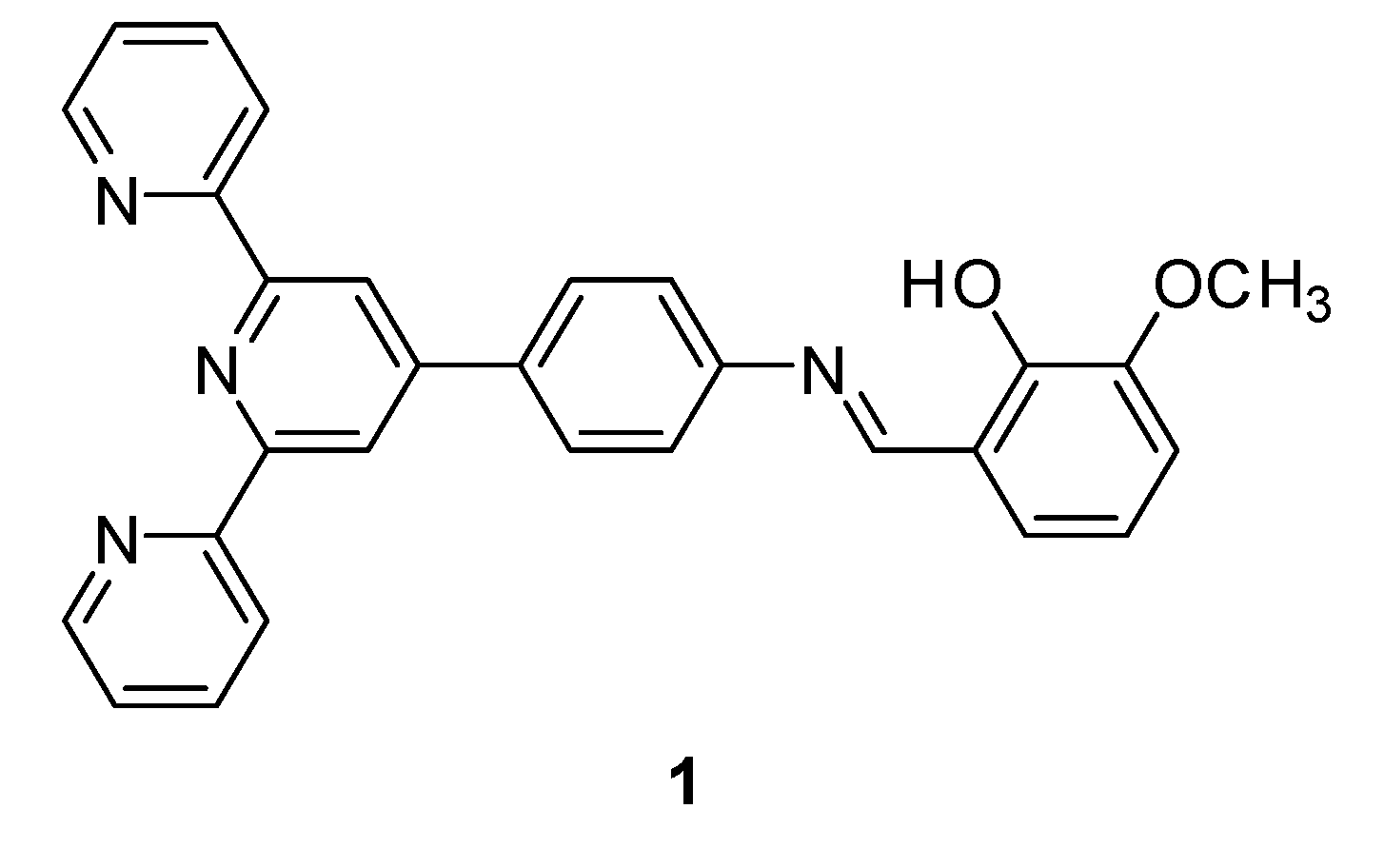
2-(((4-([2,2′:6′,2″-Terpyridin]-4′-yl)phenyl)imino)methyl)-6-methoxyphenol
Abstract
1. Introduction
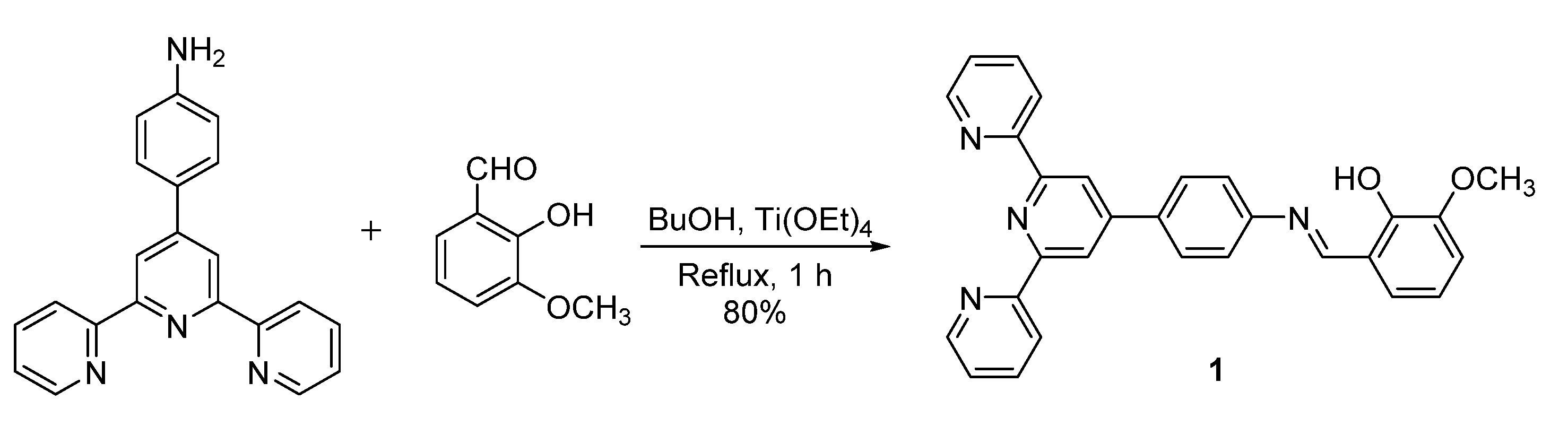
2. Results and Discussion
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cummings, S.D. Platinum complexes of terpyridine: Synthesis, structure and reactivity. Coord. Chem. Rev. 2009, 253, 449–478. [Google Scholar] [CrossRef]
- Sun, Q.; Liu, W.; Shang, W.; Zhang, H.; Xue, S.; Yang, W. Improved colorimetric dual-emission and endued piezofluorochromism by inserting a phenyl between 9-anthryl and terpyridine. Dyes Pigm. 2016, 128, 124–130. [Google Scholar] [CrossRef]
- Lisak, G.; Wagner, K.; Barnsley, J.E.; Veksha, A.; Huff, G.; Elliott, A.B.S.; Wagner, P.; Gordon, K.C.; Bobacka, J.; Wallace, G.G.; et al. Application of terpyridyl ligands to tune the optical and electrochemical properties of a conducting polymer. RSC Adv. 2018, 8, 29505–29512. [Google Scholar] [CrossRef] [PubMed]
- Balamurugan, G.; Pande, G.K.; Choi, J.-H.; Park, J.S. Enhanced electrochromic properties of terpyridine-attached asymmetric viologen with high transmittance and switching stability. Sol. Energ. Mat. Sol. C 2020, 216, 110714. [Google Scholar] [CrossRef]
- Zheng, M.; Tan, H.; Xie, Z.; Zhang, L.; Jing, X.; Sun, Z. Fast response and high sensitivity europium metal organic framework fluorescent probe with chelating terpyridine sites for Fe3+. ACS Appl. Mater. Interfaces 2013, 5, 1078–1083. [Google Scholar] [CrossRef] [PubMed]
- Karmakar, S.; Mardanya, S.; Das, S.; Baitalik, S. Efficient deep-blue emittier and molecular-scale memory device based on dipyridylephenylimidazoleeterpyridine assembly. J. Phys. Chem. C 2015, 119, 6793–6805. [Google Scholar] [CrossRef]
- Tan, J.Y.; Li, R.; Li, D.D.; Zhang, Q.; Li, S.L.; Zhou, H.P.; Yang, J.X.; Wu, J.Y.; Tian, Y.P. Thiophene-based terpyridine and its zinc halide complexes: Third-order nonlinear optical properties in the near-infrared region. Dalt. Trans. 2015, 44, 1473–1482. [Google Scholar] [CrossRef] [PubMed]
- Bhowmik, S.; Ghosh, B.N.; Marjomäki, V.; Rissanen, K. Nanomolar pyrophosphate detection in water and in a self-assembled hydrogel of a simple terpyridine-Zn2+ complex. J. Am. Chem. Soc. 2014, 136, 5543–5546. [Google Scholar] [CrossRef] [PubMed]
- Zych, D.; Slodek, A.; Matussek, M.; Filapek, M.; Szafraniec-Gorol, G.; Maślanka, S.; Krompiec, S.; Kotowicz, S.; Schab-Balcerzak, E.; Smolarek, K.; et al. 4’-Phenyl-2,2’:6’,2’’-terpyridine derivatives-synthesis, potential application and the influence of acetylene linker on their properties. Dyes Pigm. 2017, 146, 331–343. [Google Scholar] [CrossRef]
- Zeng, Y.T.; Jiang, X.; Yu, H.; Cao, Y.; Lai, Z.W.; Shi, J.J.; Wang, M. Coordination-driven terpyridine-based twisted prisms with tunable emissions and hierarchical self-assembly properties. Adv. Opt. Mater. 2022, 10, 2102613. [Google Scholar] [CrossRef]
- Du, J.; Huang, Z.; Yu, X.-Q.; Pu, L. Highly selective fluorescent recognition of histidine by a crown ether-terpyridine-Zn(II) sensor. Chem. Commun. 2013, 49, 5399–5401. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Chen, W.; Wang, L.; Yu, X.-Q.; Huang, D.-S.; Pu, L. Synthesis of a C1 symmetric BINOL–terpyridine ligand and highly enantioselective methyl propiolate addition to aromatic aldehydes. Tetrahedron 2010, 66, 1990–1993. [Google Scholar] [CrossRef]
- Chao, D.B.; Zhang, Y.X. Aggregation enhanced luminescent detection of homocysteine in water with terpyridine-based Cu2+ complexes. Sens. Actuators B 2017, 245, 146–155. [Google Scholar] [CrossRef]
- Yasuo, T.; Tohru, K.; Miyuki, E.; Keita, T.; Nagatoshi, N.; Masahiro, A. NucleophiIic reaction upon electron-deficient pyridone derivatives. X. one-pot synthesis of 3-nitropyridines by ring transformation of 1-methyl-3,5-dinitro-2-pyridone with ketones or aldehydes in the presence of ammonia. Bul1. Chem. Soc. Jpn. 1990, 63, 2820–2827. [Google Scholar]
- Shepelenko, E.N.; Podshibyakin, V.A.; Revinskii, Y.V.; Tikhomirova, K.S.; Popov, L.D.; Dubonosov, A.D.; Shcherbakov, I.N.; Bren, V.A.; Minkin, V.I. Bifunctional terpyridine/o-hydroxyimine chemosensors. J. Mol. Struct. 2018, 1154, 219–224. [Google Scholar] [CrossRef]
- Gröger, G.; Meyer-Zaika, W.; Böttcher, C.; Gröhn, F.; Ruthar, C.; Schmuck, C. Switchable supramolecular polymers from the self-assembly of a small monomer with two orthogonal binding interactions. J. Am. Chem. Soc. 2011, 133, 8961–8971. [Google Scholar] [CrossRef] [PubMed]
- Lainé, P.; Bedioui, F.; Ochsenbein, P.; Marvaud, V.; Bonin, M.; Amouyal, E. A new class of functionalized terpyridyl ligands as building blocks for photosensitized supramolecular architectures. Synthesis, structural, and electronic characterizations. J. Am. Chem. Soc. 2002, 124, 1364–1377. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, L.; Wang, J.; Gong, T.; Liu, K.; Li, J. 2-(((4-([2,2′:6′,2″-Terpyridin]-4′-yl)phenyl)imino)methyl)-6-methoxyphenol. Molbank 2023, 2023, M1664. https://doi.org/10.3390/M1664
Li L, Wang J, Gong T, Liu K, Li J. 2-(((4-([2,2′:6′,2″-Terpyridin]-4′-yl)phenyl)imino)methyl)-6-methoxyphenol. Molbank. 2023; 2023(2):M1664. https://doi.org/10.3390/M1664
Chicago/Turabian StyleLi, Lingsen, Jingjing Wang, Tianhao Gong, Kunming Liu, and Juanhua Li. 2023. "2-(((4-([2,2′:6′,2″-Terpyridin]-4′-yl)phenyl)imino)methyl)-6-methoxyphenol" Molbank 2023, no. 2: M1664. https://doi.org/10.3390/M1664
APA StyleLi, L., Wang, J., Gong, T., Liu, K., & Li, J. (2023). 2-(((4-([2,2′:6′,2″-Terpyridin]-4′-yl)phenyl)imino)methyl)-6-methoxyphenol. Molbank, 2023(2), M1664. https://doi.org/10.3390/M1664





