Abstract
A promising imidazole compound was synthesized through the following route. The reaction of 5-methyl-2-phenyl-1H-imidazole-4-carbohydrazide (1) with carbon disulfide and potassium hydroxide in ethanol (80%) afforded potassium salt (2). Refluxing of (2) with phenacyl bromide (3) in ethanol/water (1:1) gave 2-((5-(5-methyl-2-phenyl-1H-imidazol-4-yl)-1,3,4-oxadiazol-2-yl)thio)-1-phenylethan-1-one (4) in a 76% yield. The structure of the title heterocycle (4) was confirmed by single-crystal X-ray diffraction and nuclear magnetic resonance.
1. Introduction
Hydrazide derivatives display a wide variety of biological activities. For example, they have antibacterial, antitubercular, antifungal, anticancer, anti-inflammatory, anticonvulsant, antiviral, and antiprotozoal activities [1,2,3].
Imidazole is the basic core of some natural products such as histidine-, purine-, histamine and DNA-based structures [4].
Imidazole compounds showed different biological activities such as antibacterial, antimycobacterial, anti-inflammatory, antitumor, antidiabetic, anti-allergic, antipyretic, antiviral, antioxidant, anti-amoebic, antihelmintic, antifungal and ulcerogenic activities [5,6,7,8]. In addition, the 1,3,4-oxadiazole nucleus is a biologically imperative scaffold that possesses numerous biological activities, especially in the treatment of cancer disease [9,10,11].
Therefore, the current work reports the synthesis of imidazole containing 1,3,4-oxadiazole moiety using a simple procedure. Recently, the synthesis and structure elucidation of new heterocycles were reported [12,13,14,15].
2. Results and Discussion
2.1. Synthesis
The stirring of 5-methyl-2-phenyl-1H-imidazole-4-carbohydrazide (1) with potassium hydroxide and carbon disulfide in ethanol (80%) gave potassium 2-(5-methyl-2-phenyl-1H-imidazole-4-carbonyl)hydrazine-1-carbodithioate (3). Refluxing of the potassium salt (2) with phenacyl bromide (3) in aqueous ethanol (1:1) for 3 h gave 2-((5-(5-methyl-2-phenyl-1H-imidazol-4-yl)-1,3,4-oxadiazol-2-yl)thio)-1-phenylethan-1-one (4) (Scheme 1). Crystallization of the solid obtained from dimethylformamide (DMF) gave 4 in a 76% yield.
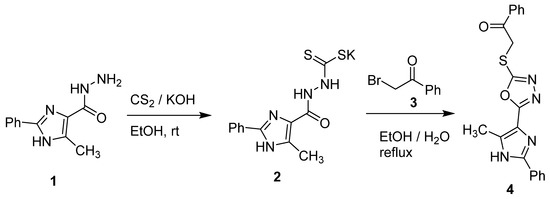
Scheme 1.
Synthesis of heterocycle 4.
2.2. NMR Spectroscopy
The 1H NMR spectrum of 4 showed the methylene protons (CH2) as a singlet signal at 5.11 ppm and the presence of an exchangeable singlet signal that appeared at 12.97 ppm due to the NH proton. The 13C NMR spectrum of 4 showed that the methylene carbon (CH2) appeared at (40.1 ppm), while the C=O carbon appeared at 193.2 ppm.
2.3. Crystal Structure Description
The title compound (4) crystalized in a monoclinic system with the P2/c space group and possessed four molecules in the unit cell. The crystal structure (Figure 1) was comprised mainly from 1,3,4-oxadiazole, 5-methyl-1H-imidazole and mercaptoacetaldehyde moieties attached with phenyl rings at the terminals.
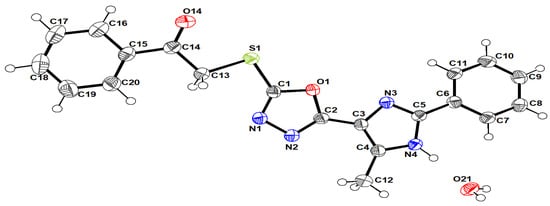
Figure 1.
A view of the structure of the title compound, showing the atom-labelling scheme. Displacement ellipsoids are drawn at a 50% probability level.
In general, the molecule geometry is in good agreement with the reported standard bond distance [16].
The whole structure generally showed planar configuration. However, torsion angles such as N3/C5/C6/C1 (9°), C1/S1/C13/C14 (170.48°) and C13/C14/C15/C20 (5.26°) showed twisting between the consisting moieties.
The structure stabilized by hydrogen bonding (Figure 2a) between the nitrogen atoms and O21, as follows: N4–H4…O21, O21–H21A…N3 and O21–H21B…N1 according to the numbering in Figure 1. The packing showed parallel layers (Figure 2b).
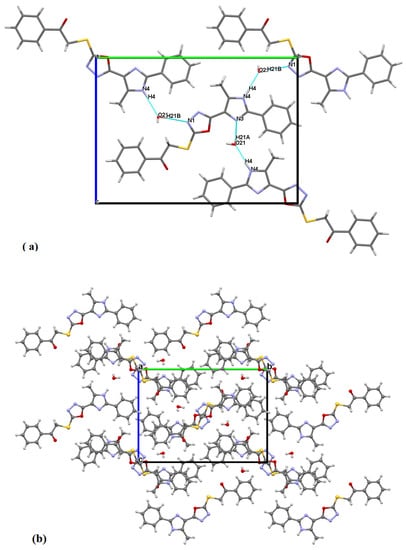
Figure 2.
(a): A segment of the structure showing hydrogen bonding as blue dotted lines. (b): Crystal packing.
3. Materials and Methods
3.1. General
Chemicals and solvents were obtained from Merck. The NMR spectra (500 MHz for 1H and 125 MHz for 13C) of 4 were performed on a JEOLNMR spectrometer. The chemical shift (δ) was reported in ppm and the coupling constant (J) was measured in Hz. The NMR spectra were recorded in DMSO-d6. Literature procedures were used to prepare 1 [17].
3.2. Synthesis of 4
A mixture of 1 (0.43 g, 2.0 mmol), potassium hydroxide (0.22 g, 4.0 mmol) and EtOH 80% (15 mL) was stirred in an ice bath for 15 min. Then, carbon disulfide (5 mL) was added and stirring was continued for 4 h. The solvent was evaporated, then the formed solid placed into a round bottom flask (50 mL) compound 3 (0.4 g, 2 mmol) and (15 mL) ethanol-water (1:1) were added. The new mixture was heated under reflux conditions for 3 h. The colorless solid produced was collected by filtration. The product was washed with EtOH, dried, and recrystallized from DMF to give 4 in a 76% yield; mp 134–135 °C. 1H NMR: 2.49 (s, 3H, Me), 5.11 (s, 2H, CH2), 7.45–8.04 (m, 10H, Ar), 12.97 (s, exch., 1H, NH). 13C NMR: 11.2, 40.1, 125.5, 127.5, 128.7, 129.0, 129.2, 129.3, 129.4, 133.1, 134.4, 134.5, 135.6, 146.2, 161.6, 193.2. Anal. Calcd. for C20H16N4O2S (376.43): C, 63.81; H, 4.28; N, 14.88. Found: C, 63.93; H, 4.39; N, 14.97%.
3.3. Crystal Structure Determination
A colorless crystal with approximate dimensions of 0.162 × 0.226 × 0.552 mm3 was placed and optically centered on a Bruker APEX-II CCD diffractometer (Mo-Kα radiations), the data collected through APEX 3 software package [18]. The structure was solved and refined using SHELXT [19] and SHELXL [20]. The crystallographic software packages PLATON [21], Mercury [22] and ORTEP-3 for Windows [23] were used for the analysis and graphical presentation of the structure.
The full crystallographic details are included in the Supplementary Materials (CIF and Tables S1–S4). The data have been deposited in the Cambridge Crystallographic Data Centre (CSD) with the number CCDC 2263548.
4. Conclusions
A new heterocycle containing imidazole and 1,3,4-oxadiazole moieties was synthesized with a good yield using a simple procedure. The structure of the title heterocycle was established using single-crystal X-ray diffraction and nuclear magnetic resonance spectroscopy.
Supplementary Materials
1H and 13C NMR spectrS1, CIFs, and checkcif reports for heterocycle 4.
Author Contributions
Conceptualization: A.A.F., B.F.A.-W. and A.H.E.H.; methodology: B.F.A.-W., A.A.F. and A.F.M.; X-ray crystal structures: J.C.F.; investigation: B.F.A.-W., A.A.F. and A.H.E.H.; writing—original draft preparation: B.F.A.-W., A.A.F., J.C.F., A.F.M. and A.H.E.H.; writing—review and editing: B.F.A.-W., A.A.F., J.C.F. and A.H.E.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article and the Supplementary Materials.
Acknowledgments
We thank the National Research Centre and University of California Davis for technical support.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Popiołek, Ł. Hydrazide–hydrazones as potential antimicrobial agents: Overview of the literature since 2010. Med. Chem. Res. 2017, 26, 287–301. [Google Scholar] [CrossRef] [PubMed]
- Popiołek, Ł. Updated information on antimicrobial activity of hydrazide–hydrazones. Int. J. Mol. Sci. 2021, 22, 9389. [Google Scholar] [CrossRef] [PubMed]
- Han, M.İ.; İmamoğlu, N. Design, synthesis, and anticancer evaluation of novel tetracaine hydrazide-hydrazones. ACS Omega 2023, 8, 9198–9211. [Google Scholar] [CrossRef] [PubMed]
- Siwach, A.; Verma, P.K. Synthesis and therapeutic potential of imidazole containing compounds. BMC Chem. 2021, 15, 12. [Google Scholar] [CrossRef]
- Verma, A.; Joshi, S.; Singh, D. Imidazole: Having Versatile Biological Activities. J. Chem. 2013, 2013, 329412. [Google Scholar] [CrossRef]
- Alghamdi, S.S.; Suliman, R.S.; Almutairi, K.; Kahtani, K.; Aljatli, D. Imidazole as a Promising Medicinal Scaffold: Current Status and Future Direction. Drug Des. Devel. Ther. 2021, 15, 3289–3312. [Google Scholar] [CrossRef]
- Tolomeu, H.V.; Fraga, C.A.M. Imidazole: Synthesis, Functionalization and Physicochemical Properties of a Privileged Structure in Medicinal Chemistry. Molecules 2023, 28, 838. [Google Scholar] [CrossRef]
- Pieczonka, A.M.; Strzelczyk, A.; Sadowska, B.; Mlostoń, G.; Stączek, P. Synthesis and evaluation of antimicrobial activity of hydrazones derived from 3-oxido-1H-imidazole-4-carbohydrazides. Eur. J. Med. Chem. 2013, 64, 389–395. [Google Scholar] [CrossRef]
- Vaidya, A.; Pathak, D.; Shah, K. 1,3,4-oxadiazole and its derivatives: A review on recent progress in anticancer activities. Chem. Biol. Drug Des. 2021, 97, 572–591. [Google Scholar] [CrossRef]
- Bajaj, S.; Asati, V.; Singh, J.; Roy, P.P. 1,3,4-Oxadiazoles: An emerging scaffold to target growth factors, enzymes and kinases as anticancer agents. Eur. J. Med. Chem. 2015, 97, 124–141. [Google Scholar] [CrossRef]
- Bajaj, S.; Roy, P.P.; Singh, J. 1,3,4-Oxadiazoles as Telomerase Inhibitor: Potential Anticancer Agents. Anticancer. Agents Med. Chem. 2018, 17, 1869–1883. [Google Scholar] [CrossRef]
- Abdel-Wahab, B.F.; Mohamed, H.A.; Farahat, A.A.; Kariuki, B.M.; El-Hiti, G.A. Reactivity of 4-bromoacetyl-1,2,3-triazoles towards amines and phenols: Synthesis and antimicrobial activity of novel heterocycles. Heterocycles 2022, 104, 1601–1613. [Google Scholar] [CrossRef]
- Abdel-Wahab, B.F.; Farahat, A.A.; Kariuki, B.M.; El-Hiti, G.A. (E)-1-(5-Methyl-1-(4-nitrophenyl)-1H-1,2,3-triazol-4-yl)ethan-1-one Oxime. Molbank 2023, 2023, M1593. [Google Scholar] [CrossRef]
- Kariuki, B.M.; Abdel-Wahab, B.F.; Farahat, A.A.; El-Hiti, G.A. Synthesis and structure determination of 1-(4-methoxyphenyl)-5-methyl-N’-(2-oxoindolin-3-ylidene)-1H-1,2,3-triazole-4-carbohydrazide. Molbank 2022, 2022, M1374. [Google Scholar] [CrossRef]
- Abdel-Wahab, B.F.; Farahat, A.A.; Awad, G.E.A.; El-Hiti, G.A. Synthesis and antimicrobial activity of some novel substituted 3-(thiophen-2-yl)pyrazole-based heterocycles. Lett. Drug Design Discov. 2017, 14, 1316–1323. [Google Scholar] [CrossRef]
- Orpen, A.G.; Brammer, L.; Allen, F.H.; Kennard, O.; Watson, D.G.; Taylor, R. Supplement. Tables of bond lengths determined by X-ray and neutron diffraction. Part 2. Organometallic compounds and co-ordination complexes of the d- and f-block metals. J. Chem. Soc. Dalton Trans. 1989, 12, S1–S83. [Google Scholar] [CrossRef]
- Chan, P.S. Preparation of Substituted Imidazo[1,5-d]-1,2,4-Triazin-1(2H)-Ones as Antihypertensives. US Patent US4743586A, 10 May 1988. [Google Scholar]
- Bruker. APEX III; Bruker AXS Inc.: Madison, WI, USA, 2019. [Google Scholar]
- Sheldrick, G.M. A short history of SHELX. Acta Cryst. 2008, A64, 112–122. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Cryst. 2015, C71, 3–8. [Google Scholar]
- Spek, A.L. Structure Validation in Chemical Crystallography. Acta Cryst. 2009, D65, 148–155. [Google Scholar] [CrossRef]
- Macrae, C.F.; Sovago, I.; Cottrell, S.J.; Galek, P.T.A.; McCabe, P.; Pidcock, E.; Platings, M.; Shields, G.P.; Stevens, J.S.; Towler, M.; et al. Mercury 4.0: From visualization to analysis, design and prediction. J. Appl. Crystallogr. 2020, 53, 226–235. [Google Scholar] [CrossRef]
- Farrugia, L.J. WinGX and ORTEP for Windows: An update. J. Appl. Cryst. 2012, 45, 849–854. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).