Abstract
Reaction of 3-aminobenzenesulfonic acid (HL1) with Cu(NO3)2·2.5H2O in H2O/DMF leads to the formation of the new bis(3-aminobenzenesulfonato-κN)-diaqua-bis(dimethylformamide-κO)-copper(II) complex [Cu(L1)2(DMF)2(H2O)2] (1) (HL1 = 3-aminobenzenesulfonic acid and DMF = N,N-dimethylformamide). Single crystal X-ray analysis reveals that the hexacoordinated copper(II) center adopts a distorted octahedral geometry with trans-oriented two 3-aminobenzenesulfonate ligands (L1−), two water and two dimethylformamide (DMF) molecules. Comparison of its crystal structure with that of the known bis(4-aminobenzenesulfonato-κN)diaquabis(dimethylformamide-κO)-copper(II) complex [Cu(L2)2(DMF)2(H2O)2] (2) (HL2 = 4-aminobenzenesulfonic acid) discloses that 1 and 2 are the isomers with an identical empirical formula (C18H30CuN4O10S2) and equal numbers of same coordinated solvents (DMF and water). H-bonded supramolecular structures and their corresponding topological analyses revealed a 2D with 6-connected hxl/Shubnikov plane net (3,6) for 1 and a 3D with 8-connected bcu; 8/4/c1; sqc3 net for 2, which are completely different.
1. Introduction
From the time of Alfred Werner, isomerism in coordination compounds is an important aspect [1,2,3,4,5,6,7,8,9,10,11,12,13,14]. Classical isomerism in coordination compounds can be classified mainly into two branches; structural isomerism (compounds have the same molecular formula but different atom sequences) and stereoisomerism (compounds have the same molecular formula, same sets of bonds, but differ in the relative orientation of these bonds) [1,2,3,4,5,6,7,8,9]. Other types of isomerism like Jahn-Teller isomerism [10] and supramolecular isomerism [11,12,13] have been also attracted much attention. Hydrate isomers of copper complexes of compartmental Schiff base were structurally characterized [14]. Isomeric ligands generating different compounds are also known [15]. However, ligand isomerism (compounds in which the overall isomerism results from isomerism solely within the ligand groups) has rarely been investigated [16,17] unlike all other isomers. Syntheses of compounds from isomeric ligands can bring valuable information for supramolecular chemistry and crystal engineering, what constitutes an aim of the present study.
Aminobenzenesulfonic acid [18,19,20,21,22,23,24,25,26,27,28] is a bifunctional species with three structural isomeric forms; ortho (2-amino) [18,19], meta (3-amino) [20,21,22] or para (4-amino) [23,24,25,26,27]. These isomeric forms and their derivatives are known for H-bond interactions via non-coordinated sulfonate oxygens but their different types of coordination mode to produce versatile molecular structure are not fully explored. 2-aminobenzenesulfonic acid was mainly converted to a Schiff base and further utilized to derive metal complexes [19] while 4-aminobenzenesulfonic acid was directly reacted with metal salts, producing either amine coordinated mononuclear or both amine and sulfonate coordinated polymeric species [23,24,25,26,27]. The other isomer 3-aminobenzenesulfonic acid has not been frequently used to synthesize metal complexes [20,21,22]. With the possibility to obtain a new compound with attractive molecular and supramolecular structures, we reacted 3-aminobenzenesulfonic acid (HL1) with Cu(NO3)2·2.5H2O and obtained the new bis(3-aminobenzenesulfonato-κN)-diaqua-bis(dimethylformamide-κO)-copper(II) complex [Cu(L1)2(DMF)2(H2O)2] (1) (Scheme 1). Herein, we report the synthesis, molecular and supramolecular structure of this mononuclear complex. Its relevant structural features (supramolecular structure and topology) are compared with that of the known bis(4-aminobenzenesulfonato-κN)diaquabis(dimethylformamide-κO)copper(II) complex (2) [Cu(L2)2(DMF)2(H2O)2] (HL2 = 4-aminobenzenesulfonic acid) (Chart S1, Supplementary Materials) [23].
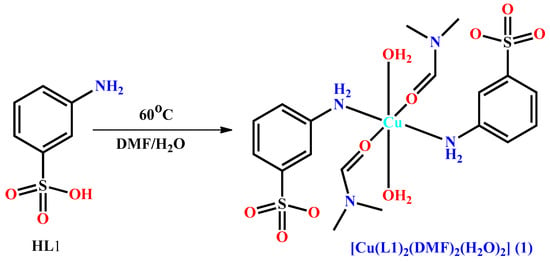
Scheme 1.
Synthesis of 1 from HL1.
2. Results and Discussion
2.1. Syntheses and Characterization
The reaction of Cu(NO3)2·2.5H2O and 3-aminobenzenesulfonic acid in aqueous DMF solution leads to the formation of the mononuclear copper(II) complex [Cu(L1)2(DMF)2(H2O)2] (1), which was characterized by elemental analysis, IR spectroscopy, thermogravimetric analysis and single crystal X-ray diffraction study (see below).
The IR spectrum of compound 1 exhibits the expected bands at 1382 cm−1 and 1216 cm−1, indicating the presence of the sulfonate group while the water and amine stretchings are observed in 3139–3538 cm−1 range.
2.2. Crystal Structure Description
An idealized ball and stick presentation of the crystal structure of the bis(3-aminobenzenesulfonato-κN)diaquabis(dimethylformamide-κO)copper(II) complex [Cu(L1)2(DMF)2(H2O)2] (1) is depicted in Figure 1, while their selected bond lengths and angles are listed in Table S1 (Supplementary Materials). Crystal structure analysis shows that it is a mononuclear copper(II) complex containing a Cu(II) center connected by trans-directed two 3-aminobenzenesulfonate ligands (L1− = 3-aminobenzenesulfonate), two water and two dimethylformamide (DMF) solvent molecules. The crystal structure is symmetric due to the presence of an inversion center. Comparison of the crystal structure with that of the reported copper(II) complex [Cu(L2)2(DMF)2(H2O)2] (2) (HL2 = 4-aminobenzenesulfonic acid) (Chart S1, Supplementary Materials) [23] reveals an interesting aspect that compounds 1 and 2 have same empirical formula (C18H30CuN4O10S2) with isomeric ligands (3-aminobenzenesulfonic acid in 1 and 4-aminobenzenesulfonic acid in 2) and the same numbers of water (two) and DMF (two) molecules, presenting them as isomers.
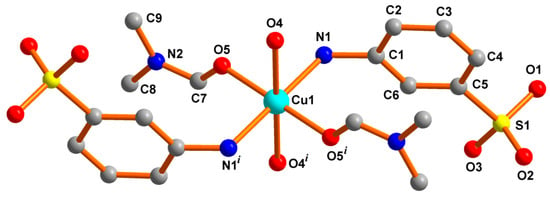
Figure 1.
Ball and stick presentation of the crystal structure of 1. All H-atoms are omitted for clarity. Symmetry: (i) 2–x, 2–y, 2–z.
Similar to compound 2, the metal center in 1 is hexacoordinated and adopts a distorted octahedral coordination environment. As shown in Figure 1, two amine nitrogen atoms (N1 and N1i) of two ligands (L1−) and two oxygen atoms (O5 and O5i) of two coordinated DMF molecules afford the basal plane for the copper(II) center, whereas the apical positions are occupied by water oxygens (O4 and O4i) alike to 2. The Cu–Namine bond distance [2.072(1) Å, Table S1] in 1 is slightly longer than the Cu–ODMF bond distance [1.971(1) Å] as in the case of 2. In contrast to the bond distances involving the four centers in the basal plane, the apical Cu–Owater bond distance is significantly longer [Cu1–O4 = 2.333(1) Å] but lower than that (2.43 Å) in 2, and the Jahn-Teller effect could be attributed for the apical elongation.
The ranges of the cisoid [86.24(6)–93.76(6)°] and transoid angles (180.00°) around the coordination environment of Cu(II) in 1 are very much comparable to those in 2 (Table S1) [23].
As shown in Figure 2 and Figure 3, there are four non-covalent H-bonding interactions in the crystal lattice of compound 1 involving water molecules (O4 or O4i), amine moieties (N1 or N1i) and non-coordinated sulfonate oxygen atoms (O1, O2, and O3). Coordinated water molecule (H4A–O4–H4B) acts as strong H-donors (dD···A = 2.79 Å and 2.82 Å and <D–H···A = 162.93° and 155.53°, Table S2) to a couple of the non-coordinated sulfonate oxygen atoms (O1iv and O2iii) of two different vicinal molecules. Similarly, coordinated amine moiety (H5–N1–H6) acts as moderate strong H-donors (dD···A = 2.90 and 3.15 Å and <D–H···A = 162.40 and 167.10°, Table S2) to two non-coordinated sulfonate oxygen atoms (O3ii and O2iii). These four interactions operate together to form an overall a 2D supramolecular structure.
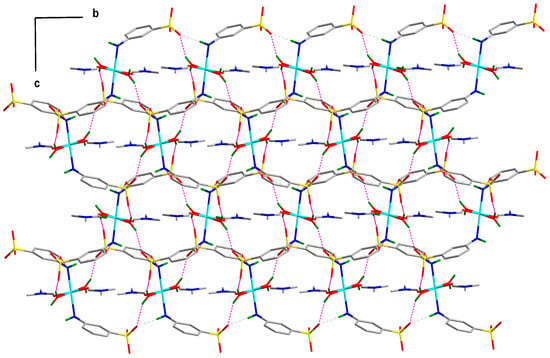
Figure 2.
Idealized wire and stick presentation (view down the a-axis and along the bc plane) of the hydrogen bonded 2D network in the lattice of 1. All H-atoms, except those participate in H-bonding interactions, are omitted for clarity.
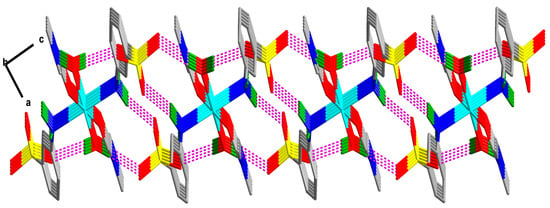
Figure 3.
View of the H-bonded 2D network in 1 down the b-axis (idealized) and along the ac-plane.
The crystal structure (Figure S1, Supplementary Materials) of 2 also contains four H-bonding interactions involving water and amine as donors and noncoordinated sulfonate as acceptor. However, co-existence of these four non-covalent interactions leads to the formation of a 3D supramolecular structure (Figure S2) unlike to the 2D motif in 1. Comparison among the geometrical parameters of H-bonds (Table S2, Supplementary Materials) in 1 and 2 demonstrates that H-bonds involving water molecules are slightly longer in 2. These longer water-sulfonate noncovalent H-bonds along with the longer metal–water covalent bonds in 2 could be the major contributing factors for the formation of the 3D network in 2, in contrast to the 2D in 1.
It may be relevant to indicate that almost 50 years ago [28], the isomeric aminobenzenesulfonate salts (ortho, para and meta) were reacted with copper(II) perchlorate and isolated copper(II) complexes were characterized by EPR, infra-red and diffuse reflectance optical spectra. Room temperature magnetic moments were also measured. As proposed by the authors, the metal ion in the p-aminobenzenesulfonate copper complex is coordinated only through the -NH2 group whereas that in ortho and meta derivatives is coordinated through both the -NH2 and -SO3 forming polymeric systems [28].
2.3. Topological Analyses
Topological analysis [29,30,31] of the hydrogen bonded networks of 1 has been also carried and compared with that of the compound 2 to illustrate their differences in the supramolecular structures. The 2D hydrogen bonded network (Figure 2 and Figure 3) in 1 can be represented as a 6-connected uninodal net (Figure 4) with topological type hxl/Shubnikov plane net (3,6), whereas the 3D hydrogen bonded network (Figure S2) of 2 can be represented as a 8-connected uninodal net with topological type bcu body centered cubic; 8/4/c1; sqc3 (Figure 4). Although, 1 and 2 are isomers but topologies of their H-bonded networks are completely different.
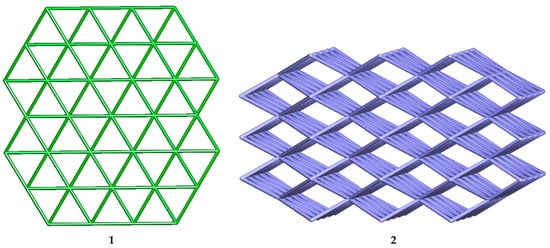
Figure 4.
Node-and-linker-type descriptions of the H-bonded 2D and 3D of 1 and 2, respectively.
2.4. Thermal Property
Thermogravimetric measurements were carried out under dinitrogen atmosphere in the range from room temperature to ca. 750 °C at a heating rate of 10 °C min−1. Features of the thermal stability of complex 1 are illustrated in Figure S3 (Supplementary Materials).
Complex 1 shows a weight loss of 6.2% in between 70 and 120 °C, corresponding to the loss of two water molecules (calcd: 6.1%). In second step, it exhibits a weight loss of 23.3% in the 130–165 °C temperature range, which accounts for the total removal of two N,N-dimethylformamide molecules (calcd: 24.6%). The remaining material is stable up to 312 °C, and beyond which it decomposes until 750 °C.
3. Experimental Section
3.1. Materials and Physical Methods
All the reagents and solvents were purchased from the commercial sources and used as received. Elemental (C, H and N) analyses were performed on a Perkin-Elmer 2400 II analyzer. IR spectra were recorded in the region 400–4000 cm−1 on a Perkin-Elmer RXIFT spectrophotometer with samples as KBr disks. Thermal properties were analyzed with a Perkin–Elmer Instruction system (STA6000) at a heating rate of 10 °Cmin−1 under dinitrogen atmosphere.
3.2. Synthesis of [Cu(L1)2(DMF)2(H2O)2] (1)
To a hot (60 °C) DMF solution (15 mL) of 3-aminobenzenesulfonic acid (HL1) (0.35 g, 2.0 mmol) was added dropwise a water solution (10 mL) of Cu(NO3)2·2.5H2O (0.23 g, 1.0 mmol). After stirring for 2 h, the resulted green solution was filtered and kept at room temperature overnight. Green crystals, suitable for X-ray diffraction analysis, were collected by filtration and washed with cold water. Yield 0.53 g (89%). Anal Calcd. for C18H30CuN4O10S2 (590): C 36.63, H 5.12, N 9.49%; Found: C 36.70, H 5.15, N 9.45%. FT-IR (cm−1, KBr): ν(H2O)/ν(N–H), 3538(br); 3238(m), 3139(m), 1618(m); ν(sulfonate), 1382(m), 1216(s), 1187(s).
3.3. Crystal Structure Determination
Diffraction data were collected on a Bruker-APEX II SMART CCD diffractometer at 296 K. For data processing and absorption correction the packages SAINT [32] and SADABS [33] were used. The structure was solved by direct and Fourier methods and refined by full-matrix least-squares based on F2 using SHELXTL [34] and SHELXL-97 [35] packages. All the hydrogen atoms except those of water molecules were inserted at calculated positions with isotropic thermal parameters and refined. Water hydrogen atoms were located from difference Fourier map. Using anisotropic treatment for the non-hydrogen atoms and isotropic treatment for the hydrogen atoms, the final refinement converged at the R1 value (I > 2σ(I)) 0.041. The crystallographic data (CCDC No. 1013699) are summarized in Table S3 (Supplementary Materials).
4. Conclusions
We have synthesized the new bis(3-aminobenzenesulfonato-κN)-diaqua-bis(dimethylformamide-κO)-copper(II) complex [Cu(L1)2(DMF)2(H2O)2] (1) by reacting 3-aminobenzenesulfonic acid (HL1) with Cu(NO3)2·2.5H2O in DMF/H2O. Comparison of its crystal structure with that of the reported 4-aminobenzenesulfonate copper(II) analogue (2) discloses an interesting fact that 1 and 2 are isomers with an identical empirical formula and equal numbers of same coordinated solvent molecules (DMF and water). Comparisons of their H-bonded networks and the corresponding topological analyses revealed that their supramolecular structures (2D in 1 and 3D in 2) and the topologies are completely different. Further investigations on the chemical and physical properties of other compounds obtained from 3-aminobenzenesulfonic acid (HL1) are ongoing.
Supplementary Materials
The following supporting information can be downloaded online. Chart S1: Chemical diagram of [Cu(L2)2(DMF)2(H2O)2] (2); Figure S1: H-bonding interactions in the asymmetric unit of 2; Figure S2: The H-bonded 3D network in 2 down the a-axis (idealized); Figure S3: Thermogravimetric curve for 1; Table S1: Comparison of selected bond lengths (Å) and angles (°) in [Cu(L1)2(DMF)2(H2O)2] (1) and [Cu(L2)2(DMF)2(H2O)2] (2). Table S2: Geometries [distances in (Å) and angles in (°)] of the H-bonds in [Cu(L1)2(DMF)2(H2O)2] (1) and [Cu(L2)2(DMF)2(H2O)2] (2). Table S3: Crystallographic data for [Cu(L1)2(DMF)2(H2O)2] (1).
Author Contributions
Conceptualization, S.H.; methodology, S.H.; software, S.H. and A.K.; validation, S.H. and A.K.; formal analysis, S.H. and A.K.; investigation, S.H. and A.K.; resources, S.H. and A.K.; data curation, S.H. and A.K.; writing—original draft preparation, S.H.; writing—review and editing, S.H. and A.K.; visualization, S.H. and A.K.; supervision, S.H.; project administration, S.H.; funding acquisition, S.H. and A.K. All authors have read and agreed to the published version of the manuscript.
Funding
S. Hazra and A. Karmakar express their gratitude to Instituto Superior Técnico (Técnico Lisboa) and FCT for Scientific Employment contracts (Contract No: IST-ID/103/2018 and IST-ID/107/2018) under Decree-Law no. 57/2016, of August 29.
Data Availability Statement
The data presented in this study are available in this article and Supplementary Materials. Crystallographic data (CCDC No. 1013699) may be downloaded from the CCDC web interface.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Sample of the compound is not available from the authors.
References
- Purcell, K.F.; Kotz, J.C. An Introduction to Inorganic Chemistry; Saunders College Publishing: Philadelphia, PA, USA, 1980. [Google Scholar]
- von Zelewsky, A. Stereochemistry of Coordination Compounds; Wiley: New York, NY, USA, 1996. [Google Scholar]
- Harvey, J.N.; Heslop, K.M.; Orpen, A.G.; Pringle, P.G. Factors controlling the relative stabilities of cis- and trans-[PtX2L2] isomers: Chatt and Wilkins—50 years on. Chem. Commun. 2003, 278–279. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, I.; Sengupta, S.; Das, S.; Banerjee, S.; Chakravorty, A. Chemistry of monovalent and bivalent rhenium: Synthesis, structure, isomer specificity and metal redox of azoheterocycle complexes. Dalton Trans. 2003, 134–140. [Google Scholar] [CrossRef]
- Haj, M.A.; Quirós, M.; Salas, J.M. Solution and solid state coexistence of head–head and head–tail isomers in dimeric Pd(ii) and Pt(ii) complexes of the type [M2(a–a)2(µ-L-N3N4)2]2+ with a bridging triazolopyrimidineligand and chelating bidentate diamines. Dalton Trans. 2002, 4740–4745. [Google Scholar] [CrossRef]
- Schaniel, D.; Woike, T.; Delley, B.; Boskovic, C.; Güdel, H.-U. Photogeneration of metastable side-on N2 linkage isomers in [Ru(NH3)5N2]Cl2, [Ru(NH3)5N2]Br2 and [Os(NH3)5N2]Cl2. Phys. Chem. Chem. Phys. 2008, 10, 5531–5538. [Google Scholar] [CrossRef]
- Bowes, K.F.; Cole, J.M.; Husheer, S.L.G.; Raithby, P.R.; Savarese, T.L.; Sparkes, H.A.; Teat, S.J.; Warren, J.E. Photocrystallographic structure determination of a new geometric isomer of [Ru(NH3)4(H2O)(η1-OSO)][MeC6H4SO3]2. Chem. Commun. 2006, 23, 2448–2450. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, A.; Fukuzawa, Y.; Chang, H.-C.; Kato, M. Vapor-Controlled Linkage Isomerization of a Vapochromic Bis(thiocyanato)platinum(II) Complex: New External Stimuli To Control Isomerization Behavior. Inorg. Chem. 2012, 51, 7508–7519. [Google Scholar] [CrossRef] [PubMed]
- Bernauer, K.; Cabort, A.; Guicher, N.; Stoeckli-Evans, H.; Süss-Fink, G. Carbonate binding to copper(II) in solution: Mixed-ligand complex formation and its application to the isolation and separation of the three isomers of [Cu(bpp)(H2O)][ClO4]2 [bpp = 2,6-bis(pyrrolidin-2-yl)pyridine]. Dalton Trans. 2002, 2069–2073. [Google Scholar] [CrossRef]
- Awaga, K.; Suzuki, Y.; Hachisuka, H.; Takeda, K. Magneto-structural correlation in the Jahn–Teller isomers of Mn12. J. Mater. Chem. 2006, 26, 2516–2521. [Google Scholar] [CrossRef]
- Ring, D.J.; Aragoni, M.C.; Champness, N.R.; Wilson, C. A coordination polymer supramolecular isomer formed from a single building block: An unexpected porphyrin ribbon constructed from zinc(tetra(4-pyridyl)porphyrin). CrystEngComm 2005, 7, 621–623. [Google Scholar] [CrossRef]
- Li, B.; Peng, Y.; Li, B.; Zhang, Y. Supramolecular isomers in the same crystal: A new type of entanglement involving ribbons of rings and 2D (4,4) networks polycatenated in a 3D architecture. Chem. Commun. 2005, 2333–2335. [Google Scholar] [CrossRef]
- Ma, C.-B.; Chen, C.-N.; Liu, Q.-T. Framework variations in Mn(ii)–organic coordination polymers: Solvent templated formation and characterisation of 1D zigzag and straight chain network isomers. CrystEngComm 2005, 7, 650–655. [Google Scholar] [CrossRef]
- Hazra, S.; Koner, R.; Nayak, M.; Sparkes, H.A.; Howard, J.A.K.; Dutta, S.; Mohanta, S. Role of Water and Solvent in the Formation of Three Mononuclear Copper(II) Crystals: A New Type of Hydrate Isomerism in Coordination Chemistry. Eur. J. Inorg. Chem. 2009, 2009, 4887–4894. [Google Scholar] [CrossRef]
- Plonka, A.M.; Banerjee, D.; Parise, J.B. Effect of Ligand Structural Isomerism in Formation of Calcium Coordination Networks. Cryst. Growth Des. 2012, 12, 2460–2467. [Google Scholar] [CrossRef]
- Coyer, M.J.; Croft, M.; Chen, J.; Herber, R.H. Ligand isomerism and stacking in square planar platinum(II) complexes. Inorg. Chem. 1992, 31, 1752–1757. [Google Scholar] [CrossRef]
- Coyer, M.J.; Herber, R.H.; Chen, J.; Croft, M.; Szu, S.P. Ligand Isomerism and Stacking in Square Planar Platinum(II) Complexes. 2. Evidence for Mixed-Isomerism in Pt[dmbp(CSN)2]. Inorg. Chem. 1994, 33, 716–721. [Google Scholar] [CrossRef]
- Tinant, B.; Robert, F.; Garcia, Y. Crystal structure of tris(1,10-phenanthroline)nickel(II) bis(o-aminobenzenesulfonate)—Ethanol (1:1), [Ni(C12H8N2)3][C6H6NO3S]2 · C2H5OH, at 120 K. Z. Kristallogr -New Cryst. Struct. 2010, 225, 546–548. [Google Scholar] [CrossRef]
- Hazra, S.; Karmakar, A.; Guedes da Silva, M.F.C.; Dlhán, L.; Boca, R.; Pombeiro, A.J.L. Dinuclear based polymeric copper(II) complexes derived from a Schiff base ligand: Effect of secondary bridging moieties on geometrical orientations and magnetic properties. Inorg. Chem. Commun. 2014, 46, 113–117. [Google Scholar] [CrossRef]
- Rubin-Preminger, J.M.; Bernstein, J. 3-Aminobenzenesulfonic Acid: A Disappearing Polymorph. Cryst. Growth Des. 2005, 5, 1343–1349. [Google Scholar] [CrossRef]
- Yuan, Y.-P.; Mao, J.-G.; Song, J.-L. Syntheses, characterizations and crystal structures of two new lead(II) amino and carboxylate–sulfonates with a layered and a pillared layered structure. J. Solid State Chem. 2004, 177, 922–927. [Google Scholar] [CrossRef]
- Brodersen, K.; Beck, R. Die Kristallstrukturen der Diquecksilber(I)-Salze von Aminobenzolsulfonsäuren. Z. Anorg. Allg. Chem. 1987, 553, 35–49. [Google Scholar] [CrossRef]
- Du, Z.-X.; Han, M.-L.; Hou, H.-W. Bis(4-aminobenzenesulfonato-κN)diaquabis(dimethylformamide-κO)copper(II). Acta Cryst. 2007, E63, m1354–m1355. [Google Scholar] [CrossRef]
- Gunderman, B.J.; Squattrito, P.J.; Dubey, S.N. Copper and Manganese Sulfanilate Hydrates. Acta Cryst. 1996, C52, 1131–1134. [Google Scholar] [CrossRef]
- Zhang, K.-J.; Meng, X.-G.; Li, X.-L. Bis(4-amino–benzene–sulfonato-κO)bis (propane-1,3-diamine-κ2N,N’)copper(II) dihydrate. Acta Cryst. 2009, E65, m1678–m1679. [Google Scholar]
- Yang, J.; Ma, J.-F.; Zhang, Y.-M.; Li, F.-F.; Liu, J.-F. Syntheses, crystal structures and characterization of divalent transition metal sulfonate complexes with O-phenanthroline. J. Coord. Chem. 2003, 56, 1409–1415. [Google Scholar] [CrossRef]
- Fu, Y.-L.; Sun, M.-N.; Zhi, X.-F.; Ng, S.W. Bis(4-amino–benzene–sulfonato-κN)bis (N,N-dimethyl–formamide-κO)copper(II). Acta Cryst. 2006, E62, m1952–m1953. [Google Scholar]
- Vinciguerra, A.; Plavidal, F.J., Jr.; Prados, R.A.; Zimmerman, R.L., Jr. Preparation, magnetic moments, infra-red and optical spectra of ortho-, meta-, and para- aminobenzenesulfonate copper(II) complexes. J. Inorg. Nucl. Chem. 1969, 31, 1061–1067. [Google Scholar] [CrossRef]
- Blatov, V.A.; Shevchenko, A.P.; Proserpio, D.M. Applied Topological Analysis of Crystal Structures with the Program Package ToposPro. Cryst. Growth Des. 2014, 14, 3576–3586. [Google Scholar] [CrossRef]
- O’Keeffe, M.; Yaghi, O.M. Reticular Chemistry Structure Resource; Arizona State University: Tempe, AZ, USA, 2005; Available online: http://rcsr.anu.edu.au/ (accessed on 1 January 2023).
- Blatov, V.A. Nanocluster analysis of intermetallic structures with the program package TOPOS. Struct. Chem. 2012, 23, 955–963. [Google Scholar] [CrossRef]
- Bruker–Nonius, APEX-II, SAINT-Plus and TWINABS; Bruker–Nonius AXS Inc.: Madison, WI, USA, 2004.
- Sheldrick, G.M. SAINT (version 6.02), SADABS (version 2.03), Bruker AXS lnc.: Madison, WI, USA, 2002.
- SHELXTL (version 6.10), Bruker AXS Inc.: Madison, WI, USA, 2002.
- Sheldrick, G.M. SHELXL-97: Crystal Structure Refinement Program; University of Göttingen: Göttingen, Germany, 1997. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).