Abstract
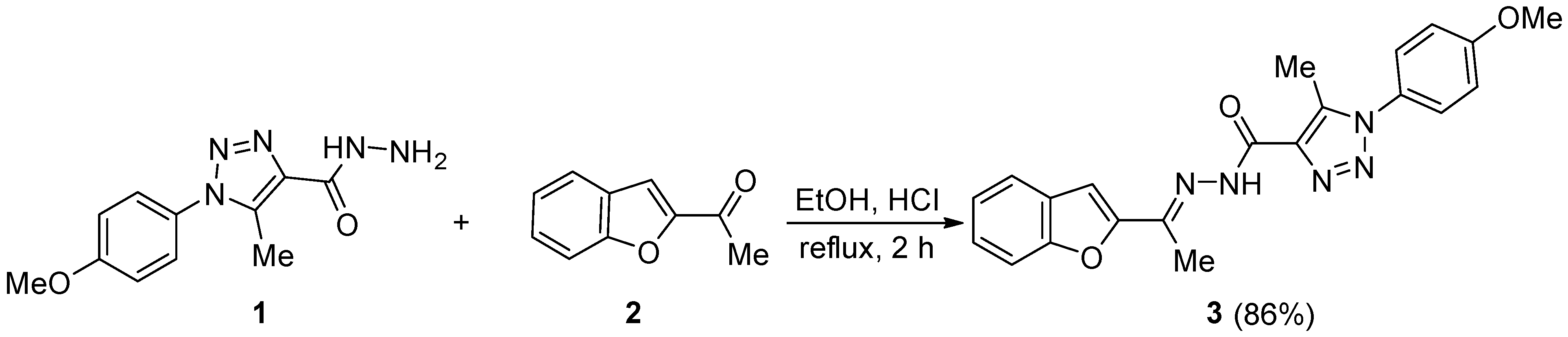
The reaction of equimolar equivalents of 1-(4-methoxyphenyl)-5-methyl-1H-1,2,3-triazole-4-carbohydrazide (1) and 2-acetylbenzofuran (2) in anhydrous ethanol containing a catalytic amount of concentrated hydrochloric acid under reflux for 2 h gave (E)-N’-(1-(benzofuran-2-yl)ethylidene)-1-(4-methoxyphenyl)-5-methyl-1H-1,2,3-triazole-4-carbohydrazide (3) in 86% yield. The structure of the title heterocycle 3 was confirmed using nuclear magnetic resonance and X-ray diffraction.
1. Introduction
Hydrazide and hydrazone derivatives display a wide variety of biological properties. For example, they have antibacterial, antitubercular, antifungal, anticancer, anti-inflammatory, anticonvulsant, antiviral, and antiprotozoal activities [1,2,3]. The main route for the synthesis of hydrazones involves reactions of appropriate acid hydrazides and carbonyl compounds (e.g., aldehydes or ketones) in alcohol (e.g., methanol, ethanol, or butanol) as the solvent [4,5,6].
Heterocycles play a central role in drug design with many medications containing heterocyclic fragments. Heterocycles can be used to manipulate drug properties, including polarity, lipophilicity, and hydrogen bonding capacity. Therefore, the pharmacological effectiveness of drugs can be optimized by modifying their heterocyclic moieties and substituents [7,8,9]. Heterocycles containing the 1,2,3-triazole moiety act as active ingredients in a variety of medications, including antibiotics (e.g., cefatrizine and tazobactam) [10]. In addition, benzofurans bearing various substituents at the C-2 position are commonly distributed in nature. They have a range of biological activities and can be used for the treatment of cardiac arrhythmias amongst other ailments [11,12,13]. An example is ailanthoidol, a natural product containing benzofuran, which shows antiviral, antioxidant, and antifungal activities [14].
The current work reports the combination of benzofuran and 1,2,3-triazole moieties in a one-step reaction using a simple procedure to synthesize a novel hydrazone. Recently, the synthesis and structure elucidation of other new heterocycles have been reported [15,16].
2. Results and Discussion
2.1. Synthesis
The condensation of 1-(4-methoxyphenyl)-5-methyl-1H-1,2,3-triazole-4-carbohydrazide (1) and an equimolar equivalent of 2-acetylbenzofuran (2) in anhydrous ethanol (EtOH) containing a catalytic amount of hydrochloric acid (HCl) under reflux for 2 h gave (E)-N’-(1-(benzofuran-2-yl)ethylidene)-1-(4-methoxyphenyl)-5-methyl-1H-1,2,3-triazole-4-carbohydrazide (3) (Scheme 1). Crystallization of the solid obtained from dimethylformamide (DMF) gave 3 in 86% yield.
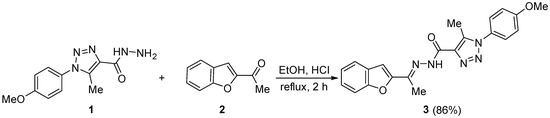
Scheme 1.
Synthesis of heterocycle 3.
2.2. IR and NMR Spectroscopy
The IR spectrum of 3 showed a strong absorption band at 1689 cm−1 due to the C=O group. The 1H NMR spectrum of 3 showed the methoxy protons as a singlet signal at 3.82 ppm and two different methyl groups (2.42 and 2.51 ppm). In addition, the spectrum showed the presence of an exchangeable singlet signal that appeared at 10.71 ppm, due to the NH proton, along with nine aromatic protons. The 13C NMR spectrum of 3 showed that the C4 of the 4-methoxylphenyl group was at a very low field (165.5 ppm). The C=O and C=N-NH carbons appeared at 162.4 and 160.0 ppm, respectively, whereas the carbon of the methoxy group appeared at a high field (60.9 ppm). See Supplementary Materials for details.
2.3. Crystal Structure of 3
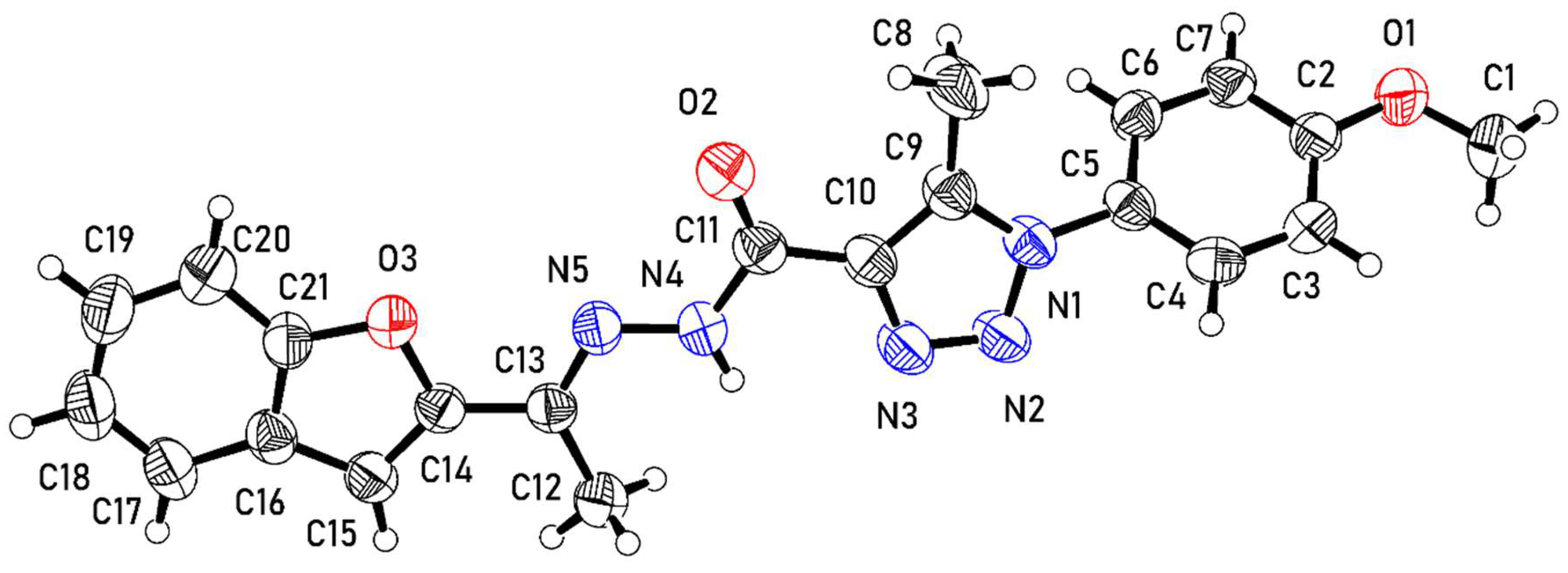
The molecule of 3 from the crystal structure is shown in Figure 1. The molecule comprises methoxybenzene (A: C1–C7, O1), methyltriazole (B: C8–C10, N1–N3), N-ethylideneformohydrazide (C: C11–C13, O2, N4, N5), and benzofuran (D: C14–C21, O3) moieties.
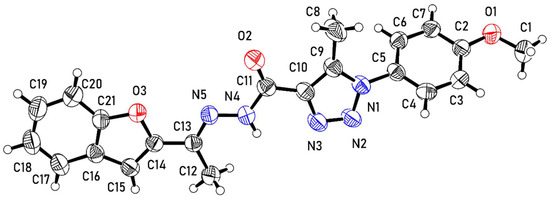
Figure 1.
An ortep representation of the molecule of 3 showing atomic displacement parameters with ellipsoids displayed at 50% probability.
In the crystal structure, groups B–D of the molecule are essentially coplanar. The twist angles of the planes through groups B–D are 7.40 (12)° and 2.21 (10)° for B/C and C/D, respectively, illustrating the co-planarity. In contrast, the plane of the methoxybenzene group (A) deviates from the plane of B–D. as indicated by an A/B twist angle of 53.94 (7)°.
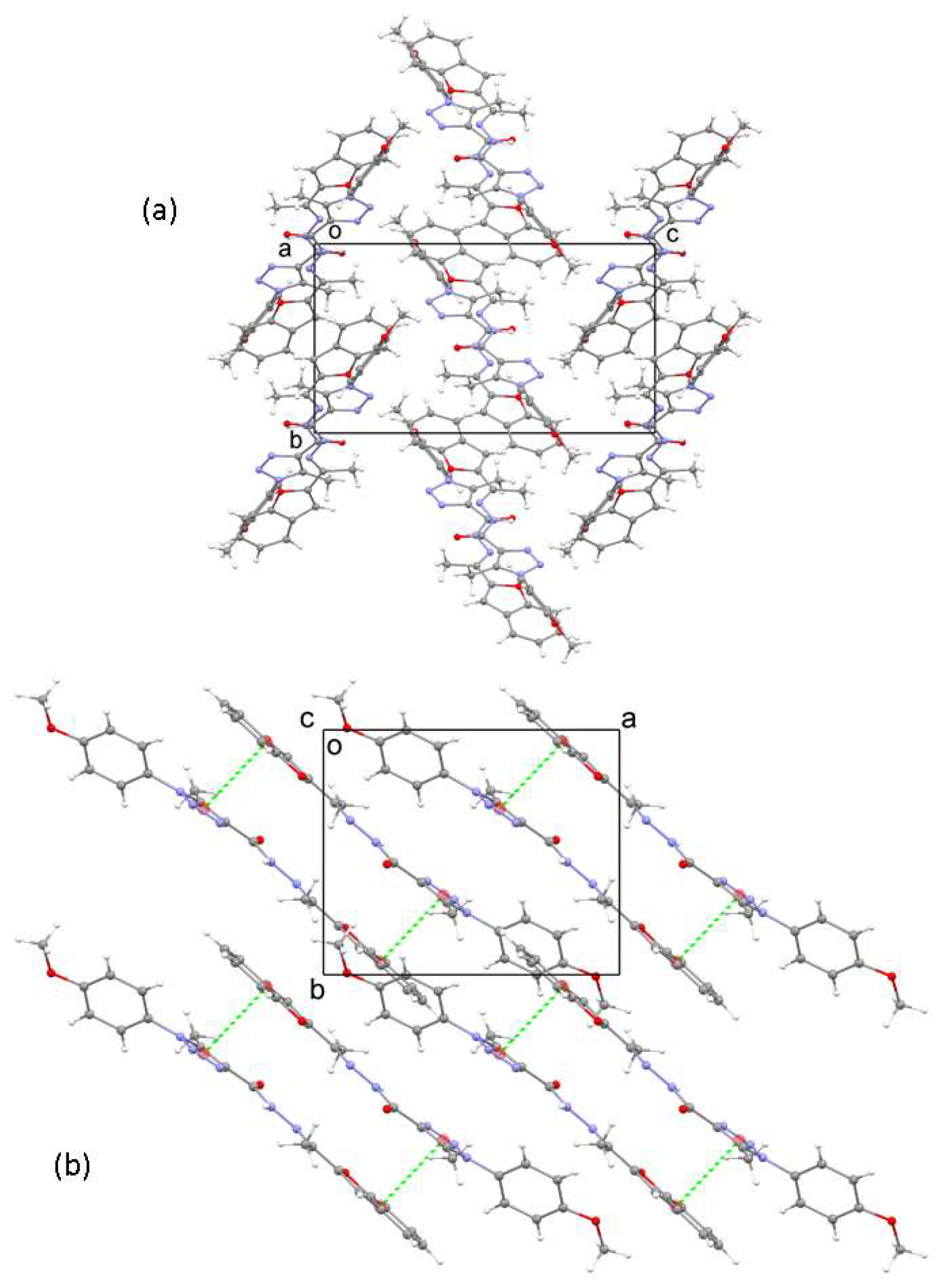
In the crystal, the molecules are arranged in layers that are oriented parallel to the ab plane (Figure 2a). Within a layer, interactions of the π–π type occur between the benzofuran and triazole rings of neighboring pairs of molecules, with centroid-to-centroid distances of 3.679 Å between the two groups (Figure 2b). The B–D planes of molecules within one layer are parallel. In contrast, the planes in neighboring layers are roughly perpendicular to each other, being oriented parallel to either (110) and (−110).
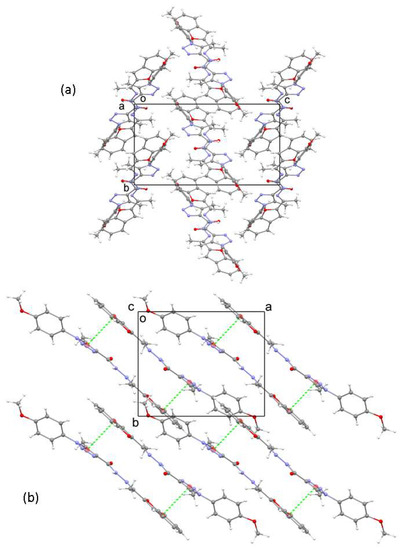
Figure 2.
(a) The crystal structure of 3 viewed down the a axis. (b) A segment of the crystal structure showing a layer with π-π interaction shown as green dashed lines. The atom colors are: grey = carbon, red = oxygen, blue = nitrogen, white = hydrogen.
3. Materials and Methods
3.1. General
Chemicals and solvents were obtained from Merck. The IR spectrum of 3 was recorded on a Bruker Vertex 80 ATR-FTIR spectrometer (400–4000 cm−1). The NMR spectra (500 MHz for 1H and 125 MHz for 13C) of 3 were obtained using a JEOLNMR spectrometer. The chemical shift (δ) is reported in ppm and coupling constant (J) was measured in Hz. The NMR spectra were recorded in DMSO-d6. Procedures from the literature were used to prepare both 1 [17] and 2 [18].
3.2. Synthesis of 3
A mixture of 1 (0.49 g, 2.0 mmol) and 2 (0.32 g, 2.0 mmol) in dry EtOH (15 mL) containing concentrated HCl (0.1 mL) was refluxed for 2 h. The mixture was left to cool to 20 °C and the colorless solid produced was collected by filtration. The product was washed with EtOH, dried, and recrystallized from DMF to give 3 in 86% yield, mp 208–210 °C. IR (KBr): 3344 (NH), 3019 (CH), 1689 (C=O), 1607 (C=C) cm−1. 1H NMR: 2.42 (s, 3H, Me), 2.51 (s, 3H, Me), 3.82 (s, 3H, OMe), 7.14 (d, 8.6 Hz, 2H, Ar), 7.25 (t, 7.2 Hz, 1H, Ar), 7.36 (t, 7.2 Hz, 1H, Ar), 7.45 (s, 1H, Ar), 7.54 (d, 8.6 Hz, 2H, Ar), 7.61–7.68 (m, 2H, Ar), 10.71 (s, exch., 1H, NH). 13C NMR: 14.7, 18.6, 60.9, 113.6, 116.7, 120.1, 127.1, 131.3, 132.2, 133.3, 142.4, 143.2, 150.5, 154.8, 158.7, 159.2, 160.0, 162.4, 165.5. Anal. Calcd. for C21H19N5O3 (389.41): C, 64.77; H, 4.92; N, 17.98. Found: C, 64.98; H, 5.09; N, 18.11%.
3.3. Crystal Structure Determination
An Agilent SuperNova Dual Atlas diffractometer using mirror monochromated MoKα radiation was used to collect single-crystal diffraction data. The structure was solved with direct methods using SHELXS [19] and refined using full-matrix least-squares methods on F2 with SHELXL [20]. C21H19N5O3, FW = 389.41, T = 293 (2) K, λ = 0.71073 Å, monoclinic, P21/c, a = 12.0718 (6) Å, b = 9.4295 (4) Å, c = 17.9224 (8) Å, β = 109.045 (5)°, V = 1928.45 (16) Å3, Z = 4, calculated density = 1.34 Mg/m3, absorption coefficient = 0.093 mm−1, F (000) = 816, crystal size = 0.400 × 0.240 × 0.170 mm3, reflections collected = 20,096, independent reflections =4981, R (int) = 0.0255, parameters = 265, goodness-of-fit on F2 = 1.056, R1 = 0.0548, wR2 = 0.1474 for (I > 2sigma (I)), R1 = 0.0827, wR2 = 0.1658 for all data, and largest difference peak and hole = 0.213 and −0.194 e.Å−3. The X-ray crystallographic data for compound 3 have been deposited in the Cambridge Crystallographic Data Center with CCDC reference number 2260659.
4. Conclusions
A new heterocycle containing 1,2,3-triazole and benzofuran moieties was synthesized with excellent yield using a simple procedure. The structure of the title heterocycle was established using X-ray diffraction and nuclear magnetic resonance spectroscopy.
Supplementary Materials
The following are available online. IR, 1H, and 13C NMR spectra, CIF, and checkcif report for heterocycle 3.
Author Contributions
Conceptualization: G.A.E.-H. and B.M.K.; methodology: B.F.A.-W., B.M.K., and G.A.E.-H.; X-ray crystal structures: B.M.K.; investigation: B.F.A.-W., M.S.B., B.M.K. and G.A.E.-H.; writing—original draft preparation: B.F.A.-W., B.M.K., M.S.B. and G.A.E.-H.; writing—review and editing: B.F.A.-W., B.M.K., M.S.B. and G.A.E.-H. All authors have read and agreed to the published version of the manuscript.
Funding
G.A.E.-H. acknowledges the support received from Researchers Supporting Project (number RSP2023R404), King Saud University, Riyadh, Saudi Arabia.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article and the Supplementary Material.
Acknowledgments
We thank the National Research Centre and Cardiff University for technical support.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Popiołek, Ł. Hydrazide–hydrazones as potential antimicrobial agents: Overview of the literature since 2010. Med. Chem. Res. 2017, 26, 287–301. [Google Scholar] [CrossRef] [PubMed]
- Popiołek, Ł. Updated information on antimicrobial activity of hydrazide–hydrazones. Int. J. Mol. Sci. 2021, 22, 9389. [Google Scholar] [CrossRef] [PubMed]
- Han, M.İ.; İmamoğlu, N. Design, synthesis, and anticancer evaluation of novel tetracaine hydrazide-hydrazones. ACS Omega 2023, 8, 9198–9211. [Google Scholar] [CrossRef] [PubMed]
- Bala, S.; Uppal, G.; Kajal, A.; Kamboj, S.; Sharma, V. Hydrazones as promising lead with diversity in bioactivity-therapeutic potential in present scenario. Int. J. Pharm. Sci. Rev. Res. 2013, 18, 65–74. [Google Scholar]
- Popiołek, Ł.; Biernasiuk, A. Hydrazide-hydrazones of 3-methoxybenzoic acid and 4-tert-butylbenzoic acid with promising antibacterial activity against Bacillus spp. J. Enzym. Inhib. Med. Chem. 2016, 31, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Popiołek, Ł.; Stefańska, J.; Kiełczykowska, M.; Musik, I.; Biernasiuk, A.; Malm, A.; Wujec, M. Synthesis, dissociation constants, and antimicrobial activity of novel 2,3-disubstituted-1,3-thiazolidin-4-one derivatives. J. Heterocycl. Chem. 2016, 53, 393–402. [Google Scholar] [CrossRef]
- Gomtsyan, A. Heterocycles in drugs and drug discovery. Chem. Heterocycl. Compd. 2012, 48, 7–10. [Google Scholar] [CrossRef]
- Tang, X.; Song, L. Recent access to polycycles via post-Ugi reactions. Processes 2023, 11, 699. [Google Scholar] [CrossRef]
- Tang, X.; Ding, S.; Song, L.; Van der Eycken, E.V. Transition metal-catalyzed C-H activation/annulation approaches to isoindolo[2,1-b]isoquinolin-5(7H)-ones. Chem. Rec. 2023, 23, e202200255. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.-Y.; Chen, Z.-A.; Shen, Q.-K.; Quan, Z.-S. Application of triazoles in the structural modification of natural products. J. Enzym. Inhib. Med. Chem. 2021, 36, 1115–1144. [Google Scholar] [CrossRef] [PubMed]
- Metwally, M.A.; Abdel-Wahab, B.F.; El-Hiti, G.A. 2-Acetylbenzofurans: Synthesis, reactions and applications. Curr. Org. Chem. 2010, 14, 48–64. [Google Scholar] [CrossRef]
- Miao, Y.; Hu, Y.; Yang, J.; Liu, T.; Sun, J.; Wang, X. Natural source, bioactivity and synthesis of benzofuran derivatives. RSC Adv. 2019, 9, 27510–27540. [Google Scholar] [CrossRef] [PubMed]
- Khodarahmi, G.; Asadi, P.; Hassanzadeh, F.; Khodarahmi, E. Benzofuran as a promising scaffold for the synthesis of antimicrobial and antibreast cancer agents: A review. J. Res. Med. Sci. 2015, 20, 1094–1104. [Google Scholar] [CrossRef] [PubMed]
- Coşkun, D.; Tekin, S.; Sandal, S.; Coşkun, M.F. Synthesis, characterization, and anticancer activity of new benzofuran substituted chalcones. J. Chem. 2016, 2016, 7678486. [Google Scholar] [CrossRef]
- Kariuki, B.M.; Abdel-Wahab, B.F.; Mohamed, H.A.; Bekheit, M.S.; El-Hiti, G.A. Synthesis and characterization of novel 2-(1,2,3-triazol-4-yl)-4,5-dihydro-1H-pyrazol-1-yl)thiazoles and 2-(4,5-dihydro-1H-pyrazol-1-yl)-4-(1H-1,2,3-triazol-4-yl)thiazoles. Molecules 2022, 27, 8904. [Google Scholar] [CrossRef] [PubMed]
- Kariuki, B.M.; Abdel-Wahab, B.F.; Bekheit, M.S.; El-Hiti, G.A. Intermolecular interactions of 3,5-bis(4-methoxyphenyl)-4,5-dihydro-1H-pyrazole-1-carbothioamide in a cocrystal with 1,3-bis(4-methoxyphenyl)prop-2-en-1-one and dimethylformamide Solvate. Crystals 2022, 12, 663. [Google Scholar] [CrossRef]
- Dong, H.-S.; Wang, B. Synthesis of some novel 3,6-bis(1,2,3-triazolyl)-s-triazolo[3,4-b]-1,3,4-thiadiazole derivatives. J. Chin. Chem. Soc. 2005, 52, 103–108. [Google Scholar] [CrossRef]
- Elliott, E.D. The preparation and properties of 2-vinylbenzofuran. J. Am. Chem. Soc. 1952, 73, 754. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A short history of SHELX. Acta Cryst. 2008, A64, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Cryst. 2015, C71, 3–8. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).