Abstract
Parkinson’s disease (PD) is a progressive neurodegenerative disorder mainly characterized by movement dysfunction. Earlier, it was found that (1R,2R,6S)-3-methyl-6-(prop-1-en-2-yl)cyclohex-3-ene-1,2-diol (Prottremin) demonstrated antiparkinsonian activity in vivo on different animal models of PD. The paper presents synthesis of new Prottremin derivative, (1R,2R,6S)-2(4-(4-isopropylbenzyl)piperazin-1-yl)-3-methyl-6-(prop-1-en-2-yl)cyclohex-3-enol. The derivative was obtained by epoxide ring opening reaction with 1-(4-isopropylbenzyl)piperazine. The product yield was 48% after purification.
1. Introduction
Parkinson’s disease (PD) is one of the most common age-related movement disorders characterized by progressive death of nigrostriatal dopamine neurons, which leads to classical symptoms of PD including tremors, rigidity, and bradykinesia. [1]. Currently, therapy against PD is aimed at relieving symptoms for a long time. Levodopa, a direct metabolic precursor of dopamine, is the gold standard of PD treatment. Unfortunately, the drug is most effective only in the first few years of administration. Additionally, the “on-off” syndrome has been found in patients with PD taking Levodopa. During the “off” period the symptoms of PD return [2].
Earlier, it was demonstrated that Prottremin ((1R,2R,6S)-3-methyl-6-(prop-1-en-2-yl)cyclohex-3-ene-1,2-diol) possessed potent antiparkinsonian activity in vivo on MPTP (1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine), rotenone, 6-hydroxydopamine (6-OHDA), and haloperidol models of PD on mice and rats at a dose of 20 mg/kg [3,4]. Further investigations showed that change in Prottremin configuration or removal of one of its functional groups (double bonds or alcohol groups) diminishes or decreases activity [3,5]. However, the addition of thiopropyl and butyl fragment to the Prottremin molecule at position 9 allows one to obtain compounds 2 and 3 with high antiparkinsonian activity [6]. In addition, epoxydiol 4, an active metabolite of Prottremin, was synthesized. It was shown that epoxydiol 4 protected cultured dopamine neurons [7]. Recently, it was found that Prottremin derivative PA96 alleviated symptoms of haloperidol-induced PD model at dose 1 mg/kg (active dose of Prottremin is 20 mg/kg) and protected cultured dopamine neurons against spontaneous and toxin-induced death of dopamine neurons [8]. Thus, the synthesis of new derivatives and analogues of Prottremin is an important task. Here, the synthesis of new derivative of Prottremin, (1R,2R,6S)-2-(4-(4-isopropylbenzyl)piperazin-1-yl)-3-methyl-6-(prop-1-en-2-yl) cyclohex-3-en-1-ol 1, is presented (Figure 1).
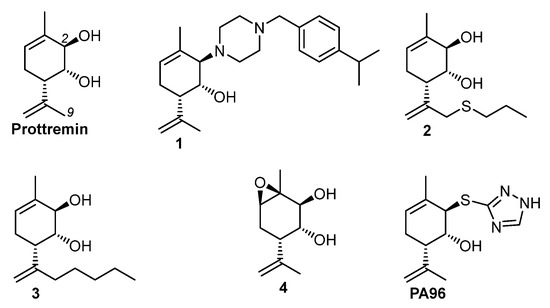
Figure 1.
Prottremin and its derivatives.
2. Results and Discussion
The synthesis of (1R,2R,6S)-2-(4-(4-isopropylbenzyl)piperazin-1-yl)-3-methyl-6-(prop-1-en-2-yl)cyclohex-3-en-1-ol 1 began with the preparation of Prottremin according to the method [3] from (−)-verbenone. The total yield of Prottremin over three stages was 23%. Thereafter, the reaction of Prottremin with acetic anhydride gave diacetate 5 [9]. After purification, diacetate 5 was refluxed with sodium tert-butoxide in toluene for 2 h affording the epoxide 6 at a 78% yield [8] (Scheme 1).
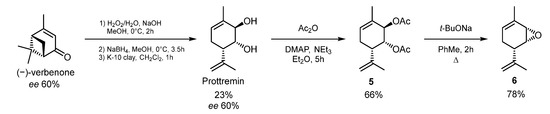
Scheme 1.
Synthesis of (1S,5S,6R)-2-methyl-5-(prop-1-en-2-yl)-7-oxabicyclo[4.1.0]hept-2-ene 6.
1-(4-Isopropylbenzyl)piperazine 9 was used for the modification of Prottremin and prepared according to [10]. This molecule contains fragments of monoterpene p-cymene demonstrating various pharmacological properties [11]. Piperazine is a versatile linker and pharmacophore for the construction of biologically active compounds [12]. At the first stage, 4-isopropylbenzaldehyde 8 reacted with tert-butyl piperazine-1-carboxylate 7 and 30 min sodium triacetoxyborohydride was added. After the work up of the reaction mixture, organic residue was involved in the reaction with trifluoroacetic acid for Boc-deprotection. The yield of product 9 was 98% (Scheme 2).
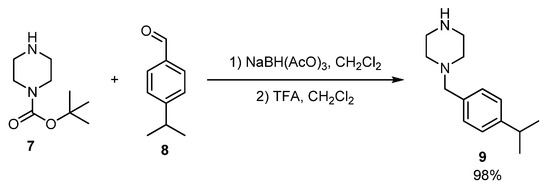
Scheme 2.
Synthesis of 1-(4-isopropylbenzyl)piperazine 9.
Reaction of epoxide 6 with 1-(4-isopropylbenzyl)piperazine 9 in the presence of potassium carbonate was carried out in ethanol. The reaction mixture was refluxed for 8 h. After purification by column chromatography on SiO2, compound 1 was isolated with 48% yield (Scheme 3).
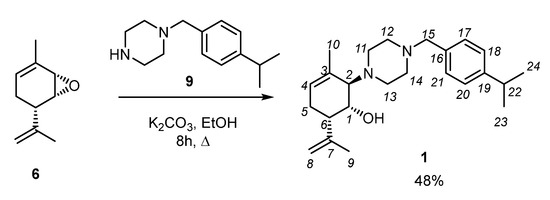
Scheme 3.
Synthesis of (1R,2R,6S)-2-(4-(4-isopropylbenzyl)piperazin-1-yl)-3-methyl-6-(prop-1-en-2-yl)cyclohex-3-en-1-ol 1.
3. Materials and Methods
3.1. General
All reagents and solvents are commercially available and used as supplied. Spectral and analytical measurements were obtained at the Multi-Access Chemical Research Center SB RAS (Novosibirsk, Russia). Column chromatography (CC): silica gel (SiO2; 60–200 μ; Macherey-Nagel); hexane/EtOAc 100:0 → 0:100 and MeOH/CH2Cl2 (1:1). GC/MS (purity control and products analysis): Agilent 7890A (Agilent Technologies, Santa Clara, CA, USA) with a quadrupole mass spectrometer Agilent 5975C as a detector, HP-5MS quartz column, 30,000 × 0.25 mm, He (1 atm) as carrier gas. Optical rotation: polAAr 3005 spectrometer (Optical Activity LTD, Huntingdon, UK), CHCl3 soln. HR-MS: DFS-Thermo-Scientific spectrometer (Thermo Fisher Scientific, Waltham, MA, USA) in a full scan mode (15–500 m/z, 70 eV electron-impact ionization, direct sample introduction) and Agilent 7200 Accurate Mass Q-TOF GC/MS (Agilent Technologies, Santa Clara, CA, USA) (70 eV, electron-impact ionization).1H- and 13C-NMR: Bruker Avance-III 600 (Bruker Corporation, Karlsruhe, Germany) apparatus at 600.30 MHz (1H) and 150.95 MHz (13C) and Bruker Avance 400 (Bruker Corporation, Karlsruhe, Germany) apparatus 400.13 MHz (1H) and 100.61 MHz (13C) in CDCl3; chemical shifts δ in ppm rel. to residual CHCl3 (δ (H) 7.24, δ (C) 76.90 ppm), J in Hz. Structure determinations: by analyzing the 1H NMR spectra, including 1H-1H 2D homonuclear correlation (COSY); J-modulated 13C NMR spectra (JMOD), and 13C-1H 2D heteronuclear correlation with one-bond and long-range spin-spin coupling constants (C-H COSY, 1J(C,H) = 135 Hz; HSQC, 1J(C,H) = 145 Hz; HMBC, 2,3J(C,H) = 7 Hz). All the target compounds reported in this paper have a purity of at least 95%. Prottremin was synthesized according to [3] from (−)-verbenone (Aldrich).
(1R,2R,6S)-3-Methyl-6-(prop-1-en-2-yl)cyclohex-3-ene-1,2-diacetate 5 was synthesized according to [9]. (1S,5S,6R)-2-Methyl-5-(prop-1-en-2-yl)-7-oxabicyclo[4.1.0]hept-2-ene 6 was synthesized from diacetate 5 according to [8].
3.2. Synthesis of 1-(4-Isopropylbenzyl)piperazine 9
4-Isopropylbenzaldehyde 8 (1.0 g, 6.75 mmol, 1 eq.) was added to the solution of tert-butyl piperazine-1-carboxylate 7 (1.26 g, 6.75 mmol, 1 eq.) in CH2Cl2 (50 mL). The reaction mixture was stirred for 30 min, and NaBH(AcO)3 (2.0 g, 9.45 mmol) was added in small portions. After 1 h the reaction mixture was washed with a saturated solution of NaHCO3 (3 × 20 mL). The organic layer was dried over Na2SO4. The desiccant was filtered off, the solvent was distilled off, the residue was dissolved in CH2Cl2 (10 mL), and TFA (3 mL) was added dropwise. The reaction mixture was stirred for 2 h, then washed with a saturated solution of K2CO3 (3 × 20 mL). The organic layer was dried over Na2SO4. The desiccant was filtered off, and the solvent was distilled off. The residue was purified by column chromatography on SiO2 with CH2Cl2/MeOH (50%) as eluent, and the product (1.4 g, 98%) was obtained as slightly yellow oil. 1H and 13C spectra are consistent with the previous results [10]. HR-MS: 218.1777 ([M+], C14H22N2; calcd 218.1778).
3.3. Synthesis of (1R,2R,6S)-2-(4-(4-Isopropylbenzyl)piperazin-1-yl)-3-methyl-6-(prop-1-en-2-yl)cyclohex-3-en-1-ol 1
Et3N (125 mg, 1.24 mmol, 1 eq.) was added to the solution of epoxide 6 (186 mg, 1.24 mmol, 1 eq.) and 1-(4-isopropylbenzyl)piperazine 9 (297 mg, 1.36 mmol, 1.1 eq.) in EtOH (10 mL). The reaction mixture was refluxed for 8 h, and then the solvent was evaporated, and the residue was diluted with EtOAc (10 mL) and washed with brine (3 × 10 mL). The organic layer was dried over Na2SO4. The desiccant was filtered off, the solvent was distilled off, and the residue was purified by column chromatography on SiO2 with EtOAc/hexane gradient (0–100%). The compound 1 (222 mg, 48%) was obtained as a slightly yellow oil. = −27.7 (c 0.39, CHCl3). 1H-NMR (CDCl3, δH, ppm, J, Hz): 1.22 (s, 3H, H-23 or H-24), 1.23 (s, 3H, H-23 or H-24), 1.53–1.62 (m, 1H, OH), 1.72 (s, 3H, H-10), 1.82 (s, 3H, H-9), 1.91 (ddm, J = 16.7, 4.6, 1H, He-5), 2.10–2.21 (m, 1H, Ha-5), 2.25 (dd, J = 11.3, 4.4, H-6), 2.41 (br. s, 4H, H-12, H-14), 2.53–2.63 (m, 2H, H-11 or H-13), 2.71–2.80 (m, 2H, H-11 or H-13), 2.87 (sept, 1H, H-22), 2.96 (br. s, 1H, H-2), 3.45 (s, 2H, H-15), 4.08 (br. s, 1H, H-1), 4.80 (br. s, 1H, H-8), 4.94 (br. s, 1H, H-8), 5.63–5.68 (m, 1H, H-4), 7.15 (d, J = 8.0, 2H, H-18, H-20), 7.21 (d, J = 8.0, 2H, H-17, H-21). 13C-NMR (CDCl3, δC, ppm): 147.42 (s; C-16), 145.99 (s; C-7), 135.23 (s; C-19), 131.15 (s; C-3), 129.11 (d; C-17, C-21), 126.04 (d; C-18, C-20), 124.78 (d; C-4), 110.97 (t; C-9), 67.85 (d; C-2), 64.97 (d; C-1), 62.86 (t; C-15), 54.03 (t; C-12, C-14), 49.10 (t; C-11, C-13), 42.62 (s; C-6), 33.60 (d; C-22), 23.88 (t; C-5), 23.88 (q; C-23, C-24), 22.85 (q; C-8), 21.85 (q; C-10). HR-MS: 368.2826 ([M+], C24H36N2O; calcd 368.2822).
1H-NMR, 13C-NMR, 2D correlation spectra 1H-1H (COSY), 1H-13C (HSQC, HMBC), and mass spectra of compound 1 are presented in Supplementary Materials.
4. Conclusions
The method for preparation of Prottremin derivative with aryl-piperazine substituent at C-2 position was developed. According to this method, (1R,2R,6S)-2-(4-(4-isopropylbenzyl)piperazin-1-yl)-3-methyl-6-(prop-1-en-2-yl)cyclohex-3-en-1-ol 1 was synthesized in 48% yield. Its structure was determined by 1D and 2D NMR experiments (HSQC, HMBC, COSY) and HRMS.
Supplementary Materials
The following supporting information can be downloaded online: Figure S1: 1H NMR spectra of compound 1 (CDCl3, 400 MHz); Figure S2. 13C NMR spectrum of compound 1 (CDCl3, 100 MHz); Figure S3. 13C NMR spectrum (JMOD) of compound 1 (CDCl3, 100 MHz); Figure S4. HSQC spectrum of compound 1 (CDCl3, 1H(400 MHz), 13C(100 MHz)); Figure S5. An expanded view of HSQC spectrum of compound 1 (CDCl3, 1H(400 MHz), 13C(100 MHz)); Figure S6. COSY spectrum of compound 1 (CDCl3, 400 MHz); Figure S7. HMBC spectrum of compound 1 (CDCl3, 1H(400 MHz), 13C(100 MHz)); Figure S8. Mass-spectrum of compound 1; Figure S9. 1H NMR spectra of compound 6 (CDCl3, 600 MHz). (*-residual solvent); Figure S10. 13C NMR spectrum (JMOD) of compound 6 (CDCl3, 150 MHz). (*-residual solvent) Figure S11. HSQC spectrum of compound 6 (CDCl3, 1H(600 MHz), 13C(150 MHz)); Figure S12. HMBS spectrum of compound 6 (CDCl3, 1H(600 MHz), 13C(150 MHz)); Figure S13. COSY spectrum of compound 6 (CDCl3, 600 MHz); Figure S14. Mass-spectrum of compound 6.
Author Contributions
Conceptualization: A.V.P., N.S.L.-Z., K.P.V. and N.F.S.; investigation: A.V.P. and N.S.L.-Z.; writing—original draft preparation: A.V.P. and N.S.L.-Z.; writing—review and editing: K.P.V. and N.F.S. All authors have read and agreed to the published version of the manuscript.
Funding
Russian state-funded project for NIOCh SB RAS (number 1021051703312-0-1.4.1).
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
The authors would like to acknowledge the Multi-Access Chemical Service Centre SB RAS for spectral and analytical measurements.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Bloem, B.R.; Okun, M.S.; Klein, C. Parkinson’s disease. Lancet 2021, 397, 2284–2303. [Google Scholar] [CrossRef] [PubMed]
- Cacabelos, R. Parkinson’s Disease: From Pathogenesis to Pharmacogenomics. Int. J. Mol. Sci. 2017, 18, 551. [Google Scholar] [CrossRef] [PubMed]
- Ardashov, O.V.; Pavlova, A.V.; Il’ina, I.V.; Morozova, E.A.; Korchagina, D.V.; Karpova, E.V.; Volcho, K.P.; Tolstikova, T.G.; Salakhutdinov, N.F. Highly Potent Activity of (1R,2R,6S)-3-Methyl-6-(prop-1-en-2-yl)cyclohex-3-ene-1,2-diol in Animal Models of Parkinson’s Disease. J. Med. Chem. 2011, 54, 3866–3874. [Google Scholar] [CrossRef] [PubMed]
- Valdmana, E.; Kapitsa, I.; Ivanova, E.; Voronina, T.; Ardashov, O.; Volcho, K.; Khazanov, V.; Salakhutdinov, N. Evolution of anti-parkinsonian activity of monoterpenoid (1R,2R,6S)-3- methyl-6-(prop-1-en-2-yl)cyclohex-3-ene-1,2-diol in various in vivo models. Eur. J. Pharmacol. 2017, 815, 351–363. [Google Scholar] [CrossRef] [PubMed]
- Ardashov, O.V.; Pavlova, A.V.; Korchagina, D.V.; Volcho, K.P.; Tolstikova, T.G.; Salakhutdinov, N.F. 3-Methyl-6-(Prop-1-En-2-Yl) Cyclohex-3-Ene-1,2-Diol: The Importance of Functional Groups for Antiparkinsonian Activity. Med. Chem. 2013, 9, 731–739. [Google Scholar] [CrossRef] [PubMed]
- Ardashov, O.; Pavlova, A.; Korchagina, D.; Volcho, K.; Tolstikova, T.; Salakhutdinov, N. Antiparkinsonian Activity of Some 9-N-, O-, S- and C-Derivatives of 3-Methyl-6-(Prop-1-En-2-Yl) Cyclohex-3-Ene-1,2-Diol. Bioorg. Med. Chem. 2013, 21, 1082–1087. [Google Scholar] [CrossRef] [PubMed]
- Ardashov, O.V.; Pavlova, A.V.; Mahato, A.K.; Sidorova, Y.; Morozova, E.A.; Korchagina, D.V.; Salnikov, G.E.; Genaev, A.M.; Patrusheva, O.S.; Li-Zhulanov, N.S.; et al. A Novel Small Molecule Supports the Survival of Cultured Dopamine Neurons and May Restore the Dopaminergic Innervation of the Brain in the MPTP Mouse Model of Parkinson’s Disease. ACS Chem. Neurosci. 2019, 10, 4337–4349. [Google Scholar] [CrossRef] [PubMed]
- Kotlyarova, A.; Podturkina, A.V.; Pavlova, A.V.; Gorina, D.S.; Lastovka, A.V.; Ardashov, O.V.; Rogachev, A.D.; Izyurov, A.E.; Arefieva, A.B.; Kulikov, A.V.; et al. Newly Identified Monoterpenoid-Based Small Molecule Able to Support the Survival of Primary Cultured Dopamine Neurons and Alleviate MPTP-Induced Toxicity In Vivo. Molecules 2022, 27, 8286. [Google Scholar] [CrossRef] [PubMed]
- Ardashov, O.; Zarubaev, V.; Shtro, A.; Korchagina, D.; Volcho, K.; Salakhutdinov, N.; Kiselev, O. Antiviral Activity of 3-Methyl-6-(prop-1-en-2-yl) Cyclohex-3-ene-1,2-diol and Its Derivatives against Influenza A(H1N1) 2009 Virus. Lett. Drug Des. Discov. 2011, 8, 375–380. [Google Scholar] [CrossRef]
- Paul, J. Procaspase-Activating Compounds and Compositions. U.S. Patent No. US 2013/0096133 A1, 18 April 2013. Available online: https://worldwide.espacenet.com/patent/search/family/048086390/publication/US2013096133A1?q=US20130096133A1 (accessed on 28 October 2022).
- Balahbib, A.; El Omari, N.; El Hachlafi, N.; Lakhdar, F.; el Menyiy, N.; Salhi, N.; Naceiri Mrabti, H.; Bakrim, S.; Zengin, G.; Bouyahya, A. Health beneficial and pharmacological properties of p-cymene. Food Chem. Toxicol. 2021, 153, 112259. [Google Scholar] [CrossRef] [PubMed]
- Rathi, A.K.; Syed, R.; Shin, H.-S.; Patel, R.V. Piperazine derivatives for therapeutic use: A patent review (2010–present). Expert Opin. Ther. Pat. 2016, 26, 777–797. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).