4-Amino-5-benzoyl-1-benzyl-2-(4,5,6,7-tetrahydro-1H-indol-2-yl)-1H-pyrrole-3-carbonitrile
Abstract
1. Introduction
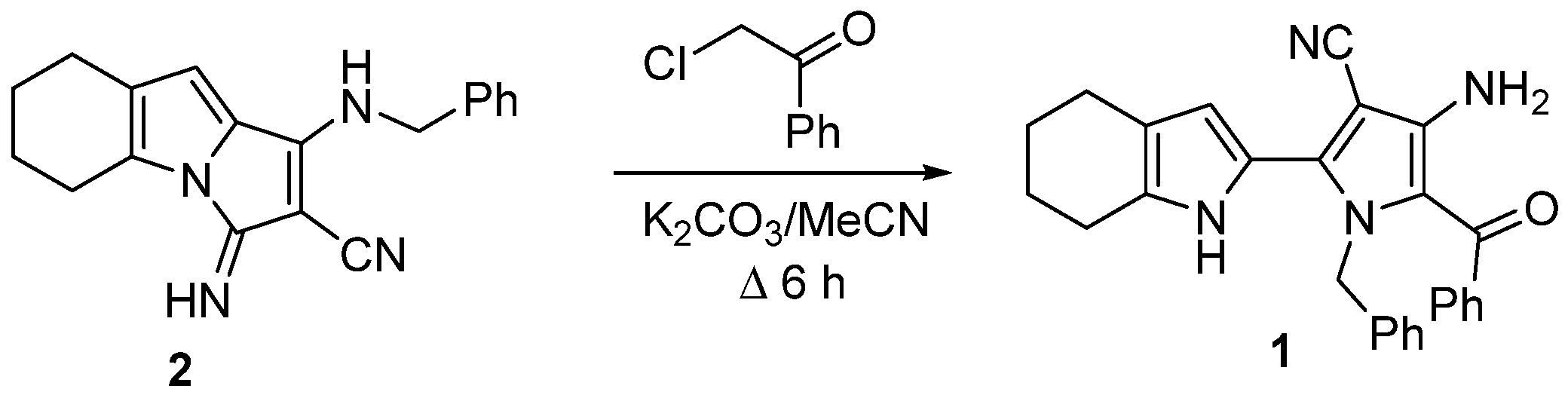
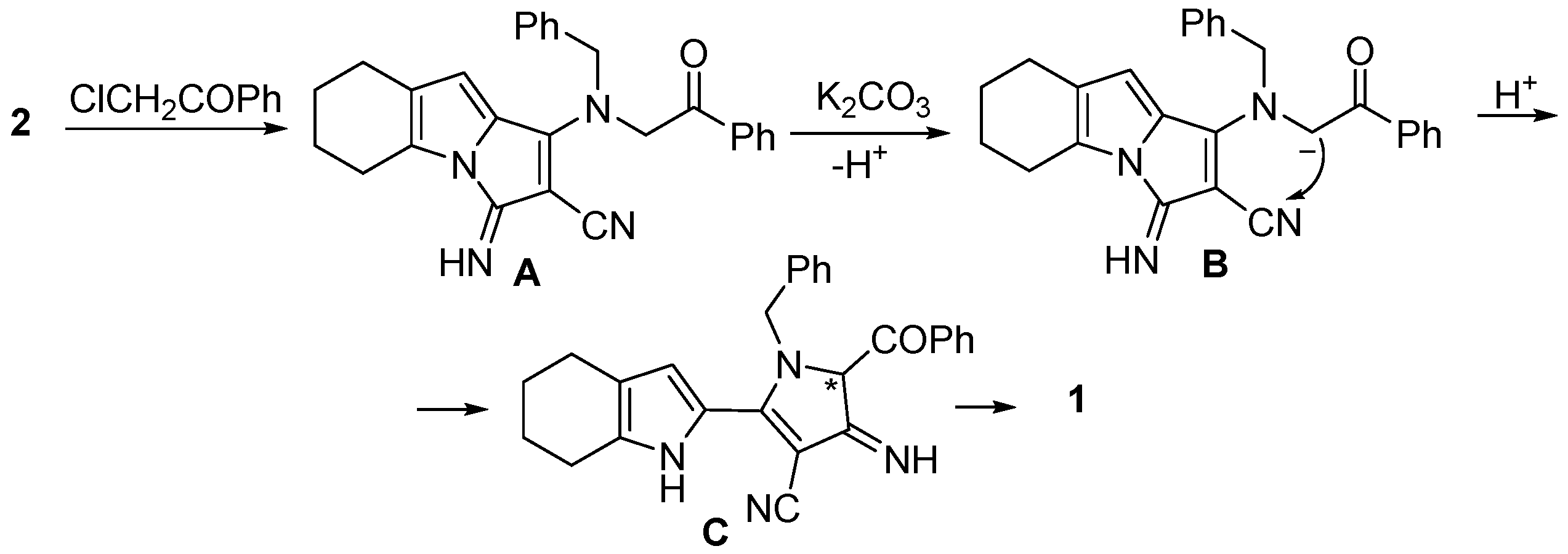
2. Result
3. Materials and Methods
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Anguera, G.; Brewster II, J.T.; Sánchez-García, D.; Sessler, J.L. Functionalized 2,2′-Bipyrroles: Building Blocks for Pyrrolic Macrocycles. Macroheterocycles 2018, 11, 227–245. [Google Scholar] [CrossRef] [PubMed]
- Islana, G.A.; Rodenak-Kladniewb, B.; Noaccoa, N.; Duranc, N.; Castro, G.R. Prodigiosin: A promising biomolecule with many potential biomedical applications. Bioengineered 2022, 13, 14227–14258. [Google Scholar] [CrossRef]
- Jiao, L.; Hao, E.; Yu, C. 200: Synthesis of 2,2′-Bipyrroles and Pyrrolyldipyrromethenes. In Handbook of Porphyrin Science; Kadish, K.M., Smith, K.M., Guilard, R., Eds.; World Scientific Publishing Co.: Singapore, 2016; Volume 41, pp. 107–164. [Google Scholar] [CrossRef]
- Setsune, J. 2,2′-Bipyrrole-Based Porphyrinoids. Chem. Rev. 2017, 117, 3044–3101. [Google Scholar] [CrossRef]
- Sessler, J.L.; Camiolo, S.; Gale, P.A. Pyrrolic and polypyrrolic anion binding agents. Coord. Chem. Rev. 2003, 240, 17–55. [Google Scholar] [CrossRef]
- Ding, Y.; Zhu, W.-H.; Xie, Y. Development of Ion Chemosensors Based on Porphyrin Analogues. Chem. Rev. 2017, 117, 2203–2256. [Google Scholar] [CrossRef]
- Naumovski, L.; Sirisawad, M.; Lecane, P.; Ramos, J.; Magda, D.; Wang, Z.; Thiemann, P.; Boswell, G.; Cho, D.-G.; Sessler, J.; et al. Development of sapphyrins as potential anticancer chemotherapy agents. Cancer Res. 2004, 64 (Suppl. S7), 475–476. [Google Scholar]
- Tong, K.-C.; Hu, D.; Wan, P.-K.; Lok, C.-N.; Che, C.-M. Anticancer Gold(III) Compounds With Porphyrin or N-heterocyclic Carbene Ligands. Front. Chem. 2020, 8, 587207. [Google Scholar] [CrossRef]
- Zhang, Q.; He, J.; Yu, W.; Li, Y.; Liu, Z.; Zhou, B.; Liu, Y. A promising anticancer drug: A photosensitizer based on the porphyrin skeleton. RSC Med. Chem. 2020, 11, 427–437. [Google Scholar] [CrossRef]
- Texidó, R.; Anguera, G.; Colominas, S.; Borrós, S.; Sánchez-García, D. Extended 2,2′-Bipyrroles: New Monomers for Conjugated Polymers with Tailored Processability. Polymers 2019, 11, 1068. [Google Scholar] [CrossRef]
- Nakamura, K.; Yasuda, N.; Maeda, H. Dimension-controlled Assemblies of Modified Bipyrroles Stabilized by Electron-withdrawing Moieties. Chem. Commun. 2016, 52, 7157–7160. [Google Scholar] [CrossRef] [PubMed]
- Hong, T.; Song, H.; Li, X.; Zhang, W.; Xie, Y. Syntheses of Mono- and Diacylated Bipyrroles with Rich Substitution Modes and Development of a Prodigiosin Derivative as a Fluorescent Zn(II) Probe. RSC Adv. 2014, 4, 6133–6140. [Google Scholar] [CrossRef]
- Saito, S.; Osuka, A. Expanded Porphyrins: Intriguing Structures, Electronic Properties, and Reactivities. Angew. Chem. Int. Ed. 2011, 50, 4342–4373. [Google Scholar] [CrossRef] [PubMed]
- Petrova, O.V.; Sagitova, E.F.; Sobenina, L.N.; Ushakov, I.A.; Borodina, T.N.; Smirnov, V.I.; Trofimov, B.A. Synthesis of functionalized 2,2′- and 2,3′-bipyrroles via 3-imino-3-H-pyrrolizine-2-carbonitriles. Tetrahedron Lett. 2016, 57, 3652–3656. [Google Scholar] [CrossRef]
- Trofimov, B.A.; Sagitova, E.F.; Petrova, O.V.; Sobenina, L.N.; Ushakov, I.A.; Vashchenko, A.V. Efficient switching from the 2,3′- to 2,2′-bipyrrole scaffold via the recyclization of 1-(benzoylmethylanilino)-3-imino-3H-2-cyanopyrrolizines: Crucial effect of the DBU organic superbase. Tetrahedron Lett. 2017, 58, 2209–2212. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petrova, O.V.; Ushakov, I.A.; Sobenina, L.N.; Kireeva, V.V.; Trofimov, B.A. 4-Amino-5-benzoyl-1-benzyl-2-(4,5,6,7-tetrahydro-1H-indol-2-yl)-1H-pyrrole-3-carbonitrile. Molbank 2023, 2023, M1547. https://doi.org/10.3390/M1547
Petrova OV, Ushakov IA, Sobenina LN, Kireeva VV, Trofimov BA. 4-Amino-5-benzoyl-1-benzyl-2-(4,5,6,7-tetrahydro-1H-indol-2-yl)-1H-pyrrole-3-carbonitrile. Molbank. 2023; 2023(1):M1547. https://doi.org/10.3390/M1547
Chicago/Turabian StylePetrova, Olga V., Igor A. Ushakov, Lyubov N. Sobenina, Victoriya V. Kireeva, and Boris A. Trofimov. 2023. "4-Amino-5-benzoyl-1-benzyl-2-(4,5,6,7-tetrahydro-1H-indol-2-yl)-1H-pyrrole-3-carbonitrile" Molbank 2023, no. 1: M1547. https://doi.org/10.3390/M1547
APA StylePetrova, O. V., Ushakov, I. A., Sobenina, L. N., Kireeva, V. V., & Trofimov, B. A. (2023). 4-Amino-5-benzoyl-1-benzyl-2-(4,5,6,7-tetrahydro-1H-indol-2-yl)-1H-pyrrole-3-carbonitrile. Molbank, 2023(1), M1547. https://doi.org/10.3390/M1547



