Abstract
Multicomponent reactions effectively contribute to modern organic and medicinal chemistry. 4-Thiazolidinone core and cyclopropyl moiety are important structural motifs for design of potential biologically active molecules. In the present paper, the convenient step-economy and cost-effective synthesis of 2-(cyclopropylamino)-5-(4-methoxybenzylidene)thiazol-4(5H)-one (2) is described based on the application of the MCR methodology. The proposed approach includes direct one-pot interaction of 2-thioxothiazolidin-4-one (rhodanine), 4-methoxybenzaldehyde with cyclopropylamine which was used in 10% excess compare to other reagents. The structure of synthesized compound 2 was confirmed using 1H, 13C, 2D NMR, LC-MS, IR and UV spectra. The presence of prototropic amino/imino tautomerism for synthesized compound 2 was observed based on spectral analysis data. Screening of antimicrobial activity against 12 strains of Gram-positive and Gram-negative bacteria, as well as yeasts, was performed for synthesized derivative 2.
1. Introduction
Multicomponent reactions (MCRs) are a highly effective tool for the synthesis of polyfunctionalized heterocyclic small molecules [1,2,3]. This approach has numerous advantages and benefits as compared to sequential multistep synthesis, namely, atom economy, efficient yields, a high bond-forming index, avoiding the use of toxic reagents, and low solvent consumption [1,2,3].
The 4-thiazolidinone core represents an important building block for the construction of multiple biologically active molecules, as well as approved drugs [4,5,6,7]. Over the past few decades, among biologically active 4-thiazolidinones scholars have identified various agents with anticancer, antibacterial, antifungal, antiviral, and antiparasitic activities. Taking into account the wide use of 4-thiazolidinone derivatives in modern drug design, further functionalization of the mentioned heterocycles with other pharmacophore fragments is an attractive area for the development of their pharmacological potential and of the search for new active agents.
The cyclopropane ring has also been the target of considerable focus in modern medicinal chemistry [8,9,10]. Due to the unique steric and electronic properties, cyclopropane fragments provide the means to obtain various potent biologically active synthetic molecules. Besides, there are numerous cyclopropyl-moiety-containing drugs approved by the FDA (Figure 1). Additionally, the cyclopropane ring can be found in bioactive natural products like terpenoids, steroids, and alkaloids.
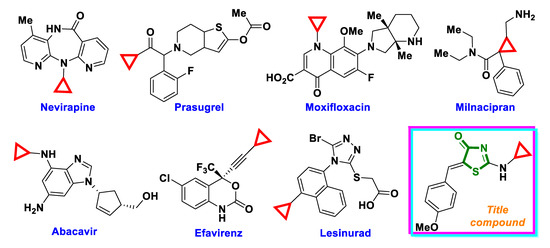
Figure 1.
Structures of cyclopropyl-moiety-containing drugs.
Taking into account all the data mentioned above and our permanent interest in the field of the new MCR protocols development for the design of novel biologically active 4-thiazolidinones [11,12,13], herein we describe the synthesis of 2-(cyclopropylamino)-5-(4-methoxybenzylidene)thiazol-4(5H)-one (2) based on the application of the MCR methodology. The results of antimicrobial activity screening against 12 strains of Gram-positive and Gram-negative bacteria, as well as yeasts for synthesized derivative 2, are presented as well.
2. Results and Discussion
2.1. Synthesis of the Title Compound 2
Two possible pathways could be proposed using the retrosynthetic approach for synthesis of title 2-(cyclopropylamino)-5-(4-methoxybenzylidene)thiazol-4(5H)-one (2) (routes A and B, Scheme 1) via two-stage protocols. However, these schemes need the application of base catalysts at least in one of the stages, and additional difficulties could be met with the separation and purification of product 1a.
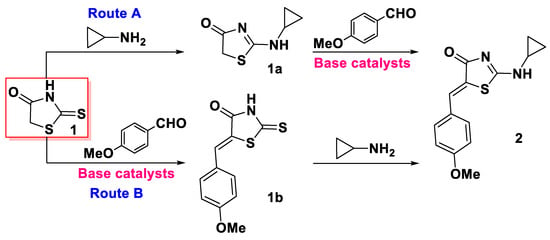
Scheme 1.
Possible retrosynthetic pathways for the synthesis of title compound 2.
That is why we developed a convenient step-economy and cost-effective multicomponent protocol for the synthesis of title compound 2. The proposed approach is based on direct one-pot interaction of 2-thioxothiazolidin-4-one (rhodanine) (1), 4-methoxybenzaldehyde (both reagents used in the equimolar amounts) with cyclopropylamine which was used in 10% excess compared to 1 and to aromatic aldehyde (Scheme 2). Application of cyclopropylamine excess allows for avoiding the additional use of base catalysts. The mixture of the above-mentioned reagents was refluxed for 4 h in the dry dioxane medium, and after cooling to room temperature, the target derivative 2 was obtained with a yield 71%. Synthesized in such a way, compound 2 possesses a good level of purity; however, it could be recrystallized from glacial acetic acid.
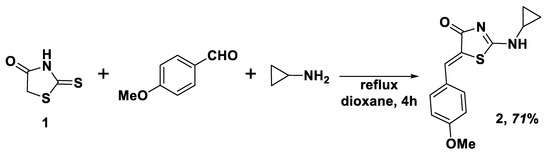
Scheme 2.
Synthesis of the title compound 2. Reagents and conditions: 2-thioxothiazolidin-4-one (1) (10 mmol), 4-methoxybenzaldehyde (10 mmol), cyclopropylamine (11 mmol), dioxane (10 mL), reflux 4 h.
The structure of synthesized compound 2 was confirmed using 1H, 13C, 2D NMR, LC-MS, IR and UV spectra (copies of spectra are presented in the Supplementary Materials).
The presence of prototropic amino/imino tautomerism for synthesized compound 2 was observed based on spectral analysis data, which is in correspondence with early-reported data for this type of heterocycles [11,14,15,16]. The possible tautomeric forms for 2 are presented on the on Figure 2.
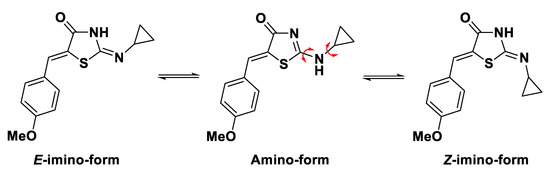
Figure 2.
Possible tautomeric forms for title compound 2.
The pattern of signals is complicated in the 1H NMR spectrum of compound 2 due to the amino/imino tautomerism. Protons of cyclopropyl moiety give a set of doubled signals at 0.67–0.62 (m), 0.72 (p), 0.80 (dt), 0.85 (dt), 2.78 (tt) and 3.07 (tt) ppm. The signal of methoxy group protons is also doubled and appeared as two singlets at 3.78 and 3.80 ppm. Aromatic protons resonate as a three multiplets at 7.04–7.11, 7.47–7.51 and 7.56–7.61 ppm. Proton of methylidene moiety gives a pair of singlets at 7.54 and 7.55 ppm. The broad signal at 9.95 ppm belongs to the proton of NH-amino-group. In the 13C NMR spectrum of compound 2, there are presented signals of all carbon atoms in the relevant areas. Carbons of cyclopropyl moiety resonate at 6.6, 7.5 (C-17, C-18), 27.7 (C-16), and 55.8, 55.9 (C-15) ppm. Carbons of 4-thiazolidinone ring give a set of signals at 126.8 (C-5), 174.5 (C-2) and 180.4 (C-4, C=O) ppm. Carbon of methylidene moiety gives a pair of singlets at 129.5 and 130.1 ppm. The molecular ion peak observed at the m/z value of 275.0 [M + H]+ in the positive ionization mode in the mass spectrum confirmed the formation of the title compound 2.
2.2. Antimicrobial Activity Evaluation In Vitro of Compound 2
The antibacterial activity of compound 2 was prescreened against 12 strains of Gram-positive and Gram-negative bacteria, as well as yeasts (Table 1). The diameter of the microbial growth inhibition zone and the minimal inhibitory concentrations (MICs) were used to assess the antibacterial activity.

Table 1.
In vitro antimicrobial activity of compound 2 (zone of growth inhibition at conc. 1 mg/mL after 24–48 h).
Compound 2 indicated differential antimicrobial property against both (clinical and reference) strains of Candida albicans and the reference strain of Raoultella ornithinolytica (Table 1).
MIC to reference Raoultella ornithinolytica and clinical Candida albicans 67 was in range 0.25 mg/mL (752.1285 μM) (Table 2).

Table 2.
MIC value (μM) of compound against bacterial species.
Based on EUCAST recommendation, MIC determination and zone diameter breakpoints for ciprofloxacin and clotrimazole sensitivity tests could be used equally justified. In our case, compound 2 MIC determination showed less efficiency (752.1285 μM) than ciprofloxacin (0.754512 μM) and clotrimazole (5.8 μM) but, using zone diameter breakpoints (disc diffusion technique), showed better results than ciprofloxacin and clotrimazole (28.3 ± 0.4 mm vs. 8.4 ± 0.4 mm for Candida albicans 67).
3. Materials and Methods
3.1. General Information and Compound 2 Synthesis
Melting points were measured in open capillary tubes on a Cole-Parmer IA9200 melting point apparatus (Antylia Scientific Ltd., Stone, UK) and are uncorrected. The elemental analyses (C, H, N) were performed using the Thermo Scientific FlashSmart Elemental Analyzer (Thermo Fisher Scientific Inc., Waltham, MA, USA) and were within ±0.4% of the theoretical values. The 600 MHz-1H and 150 MHz-13C spectra were recorded on Varian Unity Plus 600 (600 MHz) spectrometer (Varian Inc., Paulo Alto, CA, USA). All spectra were recorded at room temperature, except where indicated otherwise, and were referenced internally to solvent reference frequencies. Chemical shifts (δ) are quoted in ppm and coupling constants (J) are reported in Hz. LC–MS spectra were obtained on a Finnigan MAT INCOS-50 (Thermo Finnigan LLC, San Jose, CA, USA). The reaction mixture was monitored by thin layer chromatography (TLC) using commercial glass-backed TLC plates (Merck Kieselgel 60 F254). Solvents and reagent (Cyclopropylamine, CAS number: 765-30-0), which is commercially available, was used without further purification. The 2-thioxothiazolidin-4-one (CAS number: 141-84-4) (1) was prepared according to the protocol described in [17].
2-(Cyclopropylamino)-5-(4-methoxybenzylidene)thiazol-4(5H)-one (2)
A mixture of 10 mmol of 2-thioxothiazolidin-4-one (1), 10 mmol of 4-methoxybenzaldehyde and 11 mmol of cyclopropylamine was refluxed for 4 h in 10 mL of dioxane. After cooling to room temperature, the yellow powder was filtered off and recrystallized from acetic acid.
Yellow crystals, yield 71%, Rf = 0.80 (ethyl acetate/benzene: 1/2), mp 238–240 °C (AcOH). 1H NMR (600 MHz, DMSO-d6, δ): 0.67–0.62 (m, 1H, cyclopropyl), 0.72 (p, J = 5.0, 4.5 Hz, 1H, cyclopropyl), 0.80 (dt, J = 7.1, 3.5 Hz, 1H, cyclopropyl), 0.85 (dt, J = 7.0, 3.4 Hz, 1H, cyclopropyl), 2.78 & 3.07 (tt, J = 7.0, 3.5 Hz, 1H, cyclopropyl), 3.78 & 3.80 (s, 3H, OCH3), 7.04–7.11 (m, 2H, arom), 7.47–7.51 (m, 1H, arom), 7.54 & 7.55 (s, 1H, =CH), 7.56–7.61 (m, 1H, arom), 9.95 (brs, 1H, NH, amino). 13C NMR (150 MHz, DMSO-d6, δ): 6.6 & 7.5 (C-17, C-18), 27.7 (C-16), 55.8 & 55.9 (C-15), 115.2 & 115.3 (C-11, C-13), 126.1 (C-9), 126.8 (C-5), 129.5 & 130.1 (C-8), 131.7 & 131.8 (C-10, C-14), 160.74 (C-12), 174.5 (C-2), 180.4 (C-4). IR (KBr): 3156, 3015 (N-H), 2768 (C-H), 1678 (C=O), 1618 (C=C), 1595 (C=C) cm−1. UV–Vis (acetone) λmax (lgε, L·mol−1·cm−1): 351 (2.03), 250 (0.88) nm. LCMS (ESI+) m/z 275.0 (98.7%, [M+H]+). Anal. calc. for C14H14N2O2S: C, 61.29%; H, 5.14%; N, 10.21%. Found: C, 61.10%; H, 5.30%; N, 10.37%.
3.2. Antimicrobial Activity
Applying the agar diffusion and resazurin-based microdilution assays, the synthesized compound 2 was evaluated in vitro for its antibacterial and antifungal properties [18,19]. Vancomycin, ciprofloxacin, clotrimazole, and dimethyl sulfoxide (DMSO) were employed as a control. Reference and clinical microbial strains that had been previously identified using the 16S rRNA gene and the MALDI TOF method (Bruker, Bremen, Germany) were applied. All clinical strains were multidrug resistant with various antibiotic resistance patterns. Clinical strains were isolated from a patient at one of the nearby hospitals who had healthcare-associated infections. Testing was done three times in total.
4. Conclusions
In the present paper the multicomponent approach to the synthesis of cyclopropyl-containing 4-thiazolidinone derivative is proposed. The method is based on one-pot interaction of rhodanine, 4-methoxybenzaldehyde and cyclopropylamine and lead to the obtaining of target compound with a high yield. The synthesized title molecule is characterized by prototropic amino/imino-tautomerism. Additionally, the studied compound showed selective antimicrobial effects against reference Raoultella ornithinolytica, and a significant antifungal effect against clinical clotrimazole resistance strain Candida albicans, both of which are prominent for further studies. Obtained data contribute to the organic and medicinal chemistry of this type of heterocycle.
Supplementary Materials
Figures S1–S11: 1H, 13C, 2D NMR, LC–MS, IR and UV spectra of compound 2.
Author Contributions
Conceptualization, I.S., S.H., A.L., Y.K., V.H., A.K. and R.L.; methodology, I.S., S.H., Y.K. and R.L.; software, I.S., S.H., A.L., Y.K., S.P. and O.K.; validation, I.S., Y.K., V.H. and O.K.; investigation, I.S., S.H., A.L. and Y.K.; writing—original draft preparation, I.S., S.H., A.L., Y.K. and R.L.; writing—review and editing, I.S., S.H., A.L., Y.K. and R.L.; supervision, R.L.; project administration, R.L. All authors have read and agreed to the published version of the manuscript.
Funding
The research leading to these results received funding from the Ministry of Healthcare of Ukraine (0121U100690) and the National Research Foundation of Ukraine (2020.02/0035).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available in this article.
Acknowledgments
The authors would like to thank all the brave defenders of Ukraine who made the finalization of this article possible.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ruijter, E.; Orru, R. Synthetic and BioOrganic Chemistry Group. Multicomponent reactions in drug discovery and medicinal chemistry. Drug Discov. Today Technol. 2018, 29, 1–2. [Google Scholar] [CrossRef]
- Younus, H.A.; Al-Rashida, M.; Hameed, A.; Uroos, M.; Salar, U.; Rana, S.; Khan, K.M. Multicomponent reactions (MCR) in medicinal chemistry: A patent review (2010–2020). Expert Opin. Ther. Pat. 2021, 31, 267–289. [Google Scholar] [CrossRef] [PubMed]
- Brandão, P.; Marques, C.; Burke, A.J.; Pineiro, M. The application of isatin-based multicomponent-reactions in the quest for new bioactive and druglike molecules. Eur. J. Med. Chem. 2021, 211, 113102. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, A.C.; Gupta, S.J.; Fatima, G.N.; Sonar, P.K.; Verma, A.; Saraf, S.K. 4-Thiazolidinones: The advances continue…. Eur. J. Med. Chem. 2014, 72, 52–77. [Google Scholar] [CrossRef] [PubMed]
- Shepeta, Y.; Lozynskyi, A.; Sulyma, M.; Nektegayev, I.; Grellier, P.; Lesyk, R. Synthesis and biological activity evaluation of new thiazolidinone-diclofenac hybrid molecules. Phosphorus Sulfur Silicon Relat. Elem. 2020, 195, 836–841. [Google Scholar] [CrossRef]
- Ilkiv, I.I.; Lesyk, R.B.; Sklyarov, O.Ya. The influence of novel 4-thiazolidinone derivaties in cytoprotective mechanisms of small intestine under NSAID-induced damage. Ukr. Biochem. J. 2016, 88, 99–104. [Google Scholar] [CrossRef]
- Szczepański, J.; Tuszewska, H.; Trotsko, N. Anticancer Profile of Rhodanines: Structure-Activity Relationship (SAR) and Molecular Targets—A Review. Molecules 2022, 27, 3750. [Google Scholar] [CrossRef] [PubMed]
- Talele, T.T. The “cyclopropyl fragment” is a versatile player that frequently appears in preclinical/clinical drug molecules. J. Med. Chem. 2016, 59, 8712–8756. [Google Scholar] [CrossRef] [PubMed]
- Časar, Z. Synthetic approaches to contemporary drugs that contain the cyclopropyl moiety. Synthesis 2020, 52, 1315–1345. [Google Scholar] [CrossRef]
- Sun, M.R.; Li, H.L.; Ba, M.Y.; Cheng, W.; Zhu, H.L.; Duan, Y.T. Cyclopropyl Scaffold: A Generalist for Marketed Drugs. Mini-Rev. Med. Chem. 2021, 21, 150–170. [Google Scholar] [CrossRef]
- Golota, S.; Sydorenko, I.; Surma, R.; Karpenko, O.; Gzella, A.; Lesyk, R. Facile one-pot synthesis of 5-aryl/heterylidene-2-(2-hydroxyethyl-and 3-hydroxypropylamino)-thiazol-4-ones via catalytic aminolysis. Synth. Commun. 2017, 47, 1071–1076. [Google Scholar] [CrossRef]
- Holota, S.; Komykhov, S.; Sysak, S.; Gzella, A.; Cherkas, A.; Lesyk, R. Synthesis, Characterization and In Vitro Evaluation of Novel 5-Ene-thiazolo[3,2-b][1,2,4]triazole-6(5H)-ones as Possible Anticancer Agents. Molecules 2021, 26, 1162. [Google Scholar] [CrossRef] [PubMed]
- Lozynskyi, A.; Karkhut, A.; Polovkovych, S.; Karpenko, O.; Holota, S.; Gzella, A.K.; Lesyk, R. 3-Phenylpropanal and citral in the multicomponent synthesis of novel thiopyrano[2,3-d]thiazoles. Results Chem. 2022, 4, 100464. [Google Scholar] [CrossRef]
- Enchev, V.; Markova, N.; Angelov, S. Ab initio study of 2,4-substituted azolidines. II. Amino-imino tautomerism of 2-aminothiazolidine-4-one and 4-aminothiazolidine-2-one in water solution. J. Phys. Chem. A 2005, 109, 8904–8913. [Google Scholar] [CrossRef]
- Nowaczyk, A.; Kowiel, M.; Gzella, A.; Fijałkowski, L.; Horishny, V.; Lesyk, R. Conformational space and vibrational spectra of 2-[(2,4-dimethoxyphenyl)amino]-1,3-thiazolidin-4-one. J. Mol. Model. 2014, 20, 2366. [Google Scholar] [CrossRef]
- Türe, A.; Ergül, M.; Ergül, M.; Altun, A.; Küçükgüzel, İ. Design, synthesis, and anticancer activity of novel 4-thiazolidinone-phenylaminopyrimidine hybrids. Mol. Divers. 2021, 25, 1025–1050. [Google Scholar] [CrossRef]
- Nencki, M. Ueber die Einwirkung der Monochloressigsäure auf Sulfocyansäure und ihre Salze. J. Prakt. Chem. 1877, 16, 1–17. [Google Scholar] [CrossRef]
- Balouiri, M.; Sadiki, M.; Ibnsouda, S.K. Methods for in vitro evaluating antimicrobial activity: A review. J. Pharm. Anal. 2016, 6, 71–79. [Google Scholar] [CrossRef] [PubMed]
- EUCAST. Disk Diffusion—Manual v 10.0 (1 January 2022). Available online: https://www.eucast.org/ast_of_bacteria/disk_diffusion_methodology/ (accessed on 5 October 2022).
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).