Abstract
The title compound, 5-(1H-indol-3-yl)-1-methyl-3-phenyl-1,6-benzodiazocin-2(1H)-one, was synthesized for the first time by a one-step, two-component reaction between 1-methylbenzimidazole and benzoylindolyl-3-acetylene. The product was characterized by 1H-NMR, 13C-NMR, IR spectroscopy and HRMS.
1. Introduction
1,6-Diazocinones are outstanding representatives of the medium-size heterocyclic family of diazocines, which are of interest in the search for the central nervous system depressants, stimulants, and anti-inflammatory agents [1]. Pharmacophore groups or fragments functionalizing the diazocine ring should enhance its biological potential. For example, diazocines that are fused to the indole or pyrrole ring are cognitive enhancers [2], perform CNS-related activities [3], and they are claimed to be HIV integrase inhibitors [4].
The set of approaches for the synthesis of 1,6-diazocinones, including those that are coupled to the pyrrole ring, is limited to the reactions of the intra- [5] and intermolecular [6] cyclocondensation of the substrates containing amino and carbonyl or carboxyl groups, as well as to the ring expansion reaction of benzazepin-5-one oxime [7]. The synthesis of similar compounds from 1-(2-nitrophenyl)-1H-pyrrole-2-carbaldehyde using the strategy of the Horner–Wadsworth–Emmons reaction [8] and the intramolecular cyclocarbonylation of the aminoalkynes under the action of a palladium catalyst were described [9]. The above-mentioned approaches were implemented on single examples, which had a narrow coverage and required the use of inaccessible expensive reagents.
Earlier, while studying three-component hydrolytic opening of the benzimidazole ring under the action of acylarylacetylenes (80–82 °C, MeCN), we encountered a minor side direction: the expansion of the benzimidazole ring gave benzodiazocinones in small yields [10]. Herein, by extending this methodology to include benzoylindolyl-3-acetylene, which is readily accessible via the cross-coupling of indole with benozylbromoacetylene in solid Al2O3 [11], we report the efficient synthesis and structural characterization of 1,6-benzodiazocinone 1 which is functionalized with the indole ring.
2. Results and Discussion
5-(1H-indol-3-yl)-1-methyl-3-phenyl-1,6-benzodiazocin-2(1H)-one (1) was successfully synthesized via a one-pot two-component reaction by heating it (24 h) a MeCN solution of equimolar amounts of 1-methylbenzimidazole (2) and benzoylindolyl-3-acetylene 3 in an equimolar ratio (Scheme 1).
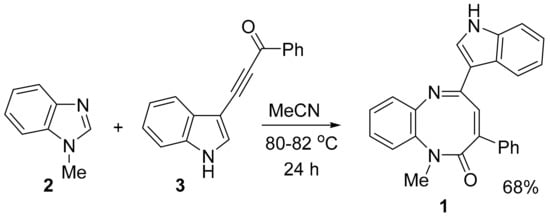
Scheme 1.
Synthesis of indolyl-1,6-benzodiazocinone 1.
The structure of the target product 1 was confirmed by the use of 1H, 13C, HMBC, HSQC and COSY NMR and IR spectroscopy (see Supplementary Materials). The HRMS and elemental analysis established the chemical formula of compound 1. The 1H NMR spectrum of 1 showed signals of the methyl group at 3.19 ppm, an olephinic proton H-4 at 6.83 ppm, and an indolyl proton at 11.84 ppm. The characteristic signals of the carbonyl and methyl groups were observed in the 13C NMR spectrum at 168.4 and 35.4 ppm, respectively. The IR spectrum reveals that there were characteristic bands of the NH group (3444 and 3159 cm−1), a C=O bond (1635 cm−1) and C=N, C=C double bonds (1573–1614 cm−1).
In conclusion, we synthesized the first representative of 1,6-benzodiazocinones which were linked to the indole ring, namely 5-(1H-indol-3-yl)-1-methyl-3-phenyl-1,6-benzodiazocin-2(1H)-one 1, which contains two pharmacologically promising counterparts and has a good chance to be useful as a drug precursor.
3. Materials and Methods
General. The NMR spectra were recorded using a Bruker DPX-400 spectrometer («Bruker», Billerica, MA, USA) (400.1 MHz for 1H and 100.6 MHz for 13C, respectively) in DMSO-d6. The internal standards were the HMDS (for 1H) and the residual solvent signals (for 13C). The coupling constants (J) were measured from the one-dimensional spectra, and the multiplicities were abbreviated as follows: s (singlet), d (doublet), dd (doublet of doublets), q (quartet), t (triplet), and m (multiplet). The IR spectra were recorded using a two-beam Bruker Vertex 70 spectrometer (Bruker Corporation, Billerica, MA, USA) in the KBr pellet. The mass spectra were recorded using an Agilent 6210 HRMS-TOF-ESI Mass spectrometer (Agilent Technologies, Santa Clara, CA, USA) in recording mode. The sample Solvent-MeCN with the addition of 0.1% heptafluorobutanoic acid included the addition of a calibration mixture for the mass spectrometer. The elemental analyses (C, H, N) were performed using an EA FLASH 1112 Series (CHN Analyzer) instrument (Thermo Finnigan, Italy). The melting points (uncorrected) were measured using Kofler micro hot-stage apparatus.
1-Methylbenzimidazole 2 and MeCN were commercial ones. Benzoylindolyl-3-acetylene 3 was obtained according to the method in [11].
Synthesis of 5-(1H-indol-3-yl)-1-methyl-3-phenyl-1,6-benzodiazocin-2(1H)-one1. A 5 mL round-bottom flask with a stir bar was sequentially charged with 1-methylbenzimidazole 2 (66 mg, 0.5 mmol), and benzoylindolylacetylene 3 (122 mg, 0.5 mmol) in MeCN (1 mL) under an atmosphere of argon. The reaction flask was capped using a glass stopper, and the reaction mixture was stirred at 80–82 °C for 24 h. Then, it was cooled to room temperature, and the precipitate was filtrated and washed with MeCN (5 × 2 mL) and Et2O (5 × 2 mL). The yield was 129 mg (68%), and it was a white powder (which formed no signal crystals, thus, it is not suitable for an X-ray analysis), with an mp of 298–300 °C. The IR spectrum (KBr), ν, in cm−1 was: 3444 (NH), 3159 (NH), 1635 (C=O), 1614, 1582, and 1573 (C=N, C=C). The result of the 1H NMR (DMSO-d6, ppm) was: 3.19 (s, 3H, Me), 6.83 (s, 1H, H-4), 7.05 (d, 3JH9,H10 = 7.9 Hz, 1H, H-10), 7.09 (t, 3JH4′,H5′ = 7.6 Hz, 3JH5′,H6′ = 7.6 Hz, 1H, H-5′), 7.20 (t, 3JH5′,H6′ = 7.6 Hz, 3JH6′,H7′ = 8.0 Hz, 1H, H-6′), 7.23–7.29 (m, 2H, H-8, H-9), 7.34–7.40 (m, 3H, Hp,m from Ph), 7.49 (d, 3JH7,H8 = 8.0 Hz, 1H, H-7), 7.51 (d, 3JH6′,H7′ = 8.0 Hz, 1H, H-7′), 7.50–7.55 (m, 2H, Ho from Ph), 8.01 (d, 3JNH,H2 = 2.8 Hz, 1H, H-2′), 8.39 (d, 3JH4′,H5′ = 7.6 Hz, 1H, H-4′), and 11.84 (s, 1H, NH). The result of the 13C{1H} NMR (DMSO-d6, ppm) was: 35.4 (Me), 112.0 (C-7′), 114.5 (C-3′), 120.3 (C-4), 121.0 (C-6′), 122.2 (C-4′), 122.5 (C-10), 122.7 (C-8), 123.9 (C-5′), 124.8 (C-3a’), 126.4 (C-7), 126.5 (Co from Ph), 127.7 (C-9), 128.6 (Cm from Ph), 128.8 (Cp from Ph), 132.3 (C-2′), 133.8 (C-10a), 134.9 (Ci from Ph), 137.1 (C-7a’), 142.0 (C-3), 148.6 (C-6a), 163.0 (C-5), and 168.4 (C-2). The result of the HRMS (ESI) which was Calcd. for C25H20N3O+ [M + H]+ were: 378.16064, which found: 378.16024. The anal. calcd. which was for C25H19N3O (%) yielded: C, 79.55; H, 5.07; N, 11.13. The amounts that were found (%) were: C, 79.47; H, 5.15; N, 11.38.
Supplementary Materials
Copies of 1H-NMR, 13C-NMR, HMBC, HSQC, COSY and HMRS spectra.
Author Contributions
Conceptualization, K.V.B.; methodology, D.N.T.; investigation, K.V.B.; data curation, K.V.B. and A.V.A.; writing—original draft preparation, K.V.B.; writing—review and editing, K.V.B.; supervision, B.A.T.; project administration, K.V.B.; funding acquisition, K.V.B. All authors have read and agreed to the published version of the manuscript.
Funding
This work was financially supported by a grant from the Russian Science Foundation (project no. 21-73-10134).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors thank the Baikal Analytical Centre of collective use and Shared Research Facilities for Physical and Chemical Ultramicroanalysis, Limnological Institute, SB RAS (HRMS-TOF Spectra) for the equipment.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Sulkowski, T.S. 8-Aryl-2,3,6,7-tetrahydro-1,4-diazocin-5(4H)-ones and Related Compounds. U.S. Patent US3293243, 20 December 1966. Chem. Abstr.1967, 66, 46444. [Google Scholar]
- Mazul’ov, A.A.; Andronati, S.A.; Korotenko, T.I.; Sokolenko, N.I.; Dyadenko, A.I.; Shapiro, Y.E.; Gorhatyuk, V.Y.; Voronina, T.A. Design of a novel cognitive enhancer (8S,10aS)-8-carbamoyl-1,2,3,6,7,8,9,10a-octahydro-5H,10H-pyrrolo[1,2-a][1,4]diazocin-5,10-dione. Bioorg. Med. Chem. Lett. 1996, 6, 2595–2600. [Google Scholar]
- Gatta, F.; Ponti, F. Synthesis of indolo-quinazolines, indolo-1,4-benzodiazepines and indolo-1,5-benzodiazocines with potential activity on CNS. Boll. Chim. Farm. 1981, 120, 102–107. [Google Scholar]
- Isaacs, R.C.A.; Wai, J.S.; Fisher, T.E. HIV Integrase inhibitors, PCT Int. Appl., WO 2009/154870 A1 20091223. Chem. Abstr. 2009, 152, 97513. [Google Scholar]
- Ivanov, Y.E.; Yavolovsky, A.A.; Mazepa, A.V.; Krasnoshchekaya, S.P. Synthesis and reactions of 1,6-dibenzoyl-5H,10H-diimidazo[1,5-a;1′,5′-d]pyrazine-5,10-dione. Chem. Heterocycl. Compd. 2003, 39, 250–254. [Google Scholar] [CrossRef]
- Velikorodov, A.V.; Stepkina, N.N.; Osipova, V.P.; Zukhairaeva, A.S.; Shustova, E.A. Synthesis of new functionally substituted indenes, benzofurans, and 2,5-benzodiazocin-1(2H)-ones. Russ. J. Org. Chem. 2021, 57, 575–581. [Google Scholar] [CrossRef]
- Kenwright, J.L.; Galloway, W.R.J.D.; Wortmann, L.; Spring, D.R. Mild and efficient synthesis of benzo-fused 7- and 8-membered ring lactams: A convenient approach towards biologically interesting chemotypes. Synth. Commun. 2013, 43, 1508–1516. [Google Scholar] [CrossRef]
- Mishra, A.; Batra, S. Expeditious synthesis of imidazole- and pyrrole-fused benzodiazocines. Eur. J. Org. Chem. 2010, 2010, 4832–4840. [Google Scholar] [CrossRef]
- Lu, S.M.; Alper, H. Sequence of intramolecular carbonylation and asymmetric hydrogenation reactions: Highly regio- and enantioselective synthesis of medium ring tricyclic lactams. J. Am. Chem. Soc. 2008, 130, 6451–6455. [Google Scholar] [CrossRef] [PubMed]
- Belyaeva, K.V.; Andriyankova, L.V.; Nikitina, L.P.; Bagryanskaya, I.Y.; Afonin, A.V.; Ushakov, I.A.; Mal’kina, A.G.; Trofimov, B.A. Reaction of 1-substituted benzimidazoles with acylacetylenes and water: Ring-opening versus ring-expansion and isotopic effect of deuterium. Tetrahedron 2015, 71, 2891–2899. [Google Scholar] [CrossRef]
- Sobenina, L.N.; Demenev, A.P.; Mikhaleva, A.I.; Ushakov, I.A.; Vasil’tsov, A.M.; Ivanov, A.V.; Trofimov, B.A. Ethynylation of indoles with 1-benzoyl-2-bromoacetylene on Al2O3. Tetrahedron Lett. 2006, 47, 7139–7141. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).