Abstract
A simple and convenient procedure for the γ, β-dimerization of verbenone was developed. The dimer was obtained during aging with KOH without a solvent. The process proceeds as the formation of the extended enolate of verbenone and its Michael addition to other molecules of verbenone. The product yield was 82% after purification by column chromatography and recrystallization.
1. Introduction
Verbenone is a natural monoterpenoid with pinene framework and plays an important role in the relationships between different species in some ecosystems. On the one hand, it is found in plants: large quantities of verbenone were observed in some rosemary oils (27.0–28.9%) [1] and it was found as major component in flowers of different chrysanthemum cultivars and their wild relatives [2]. On the other hand, verbenone is a purported anti-aggregation pheromone of several economically significant bark beetle species [3]. Moreover, the mycangial symbiotic fungus of Dendroctonus frontalis produces verbenone, thus, the development of fungus in the plant host may play a role in influencing the behavior of the beetle to a success fully colonized tree [4]. The most important way to obtain verbenone is the allylic oxidation of widespread—and available in large amounts—monoterpene α-pinene [5,6]. Verbenone as a component of essential oil can be used in aromatherapy [7]. Finally, one of the most important directions for the use of verbenone is fine chemistry. Verbenone, being a chiral compound, is used in the asymmetric synthesis of complex biologically active compounds, for example, a diol with a para-menthane skeleton with high antiparkinsonian activity [8] or the antitumor agent, taxol [9].
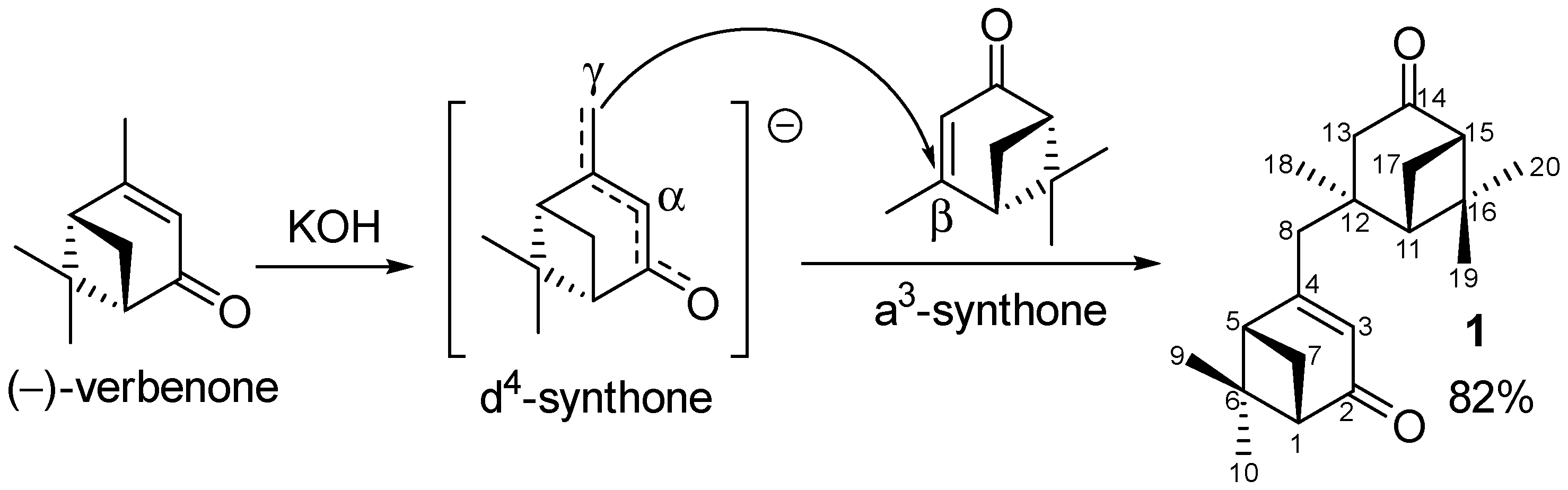
It is known that the extended enolate of verbenone can act as a d4-synthon, in particular, it reacts with aldehydes at the methyl group in the presence of a base [10,11,12]. At the same time, verbenone itself is a Michael acceptor, thus, is an a3-synthon. However, in the case of simple C-nucleophiles, this obvious approach does not work, and to obtain the corresponding products, the following detours are used: through cyclopropanation, followed by the opening of the cyclopropane ring [13]; or on the basis of nopinone, which temporarily introduces an additional SO2Ph acceptor into the α-positions, and is subsequently removed with Li/NH3 (liquid) [14]. However, verbenone can react with a strong delocalized conjugated C-nucleophile, such as a structurally similar dianion of crotonic acid. [15]. Moreover, the nucleophile, corresponding to an extended enolate, is attached at its γ-position. Therefore, verbenone can be potentially dimerized, and since in the case of verbenone dianion, it does not need to be created, the reaction should proceed under mild conditions. The resulting γ, β-dimer is promising for further modification, including the separate modification of each fragment with a pinane framework due to their structural difference. Importantly, to the best of our knowledge there are no data on the γ, β-dimerization of (−)-verbenone. Dimers of different structures obtained by the oxidation of the extended enolate of verbenone are described [16]. In particular, the γ, γ-attachment at methyl groups was observed when using CuCl2, but α, γ-joint occurred in the case of FeCl3.
2. Results and Discussion
We found that the dimerization of verbenone actually proceeds when it interacts for a week at room temperature without a solvent with KOH ground in a mortar. The yield of product 1 (Scheme 1) after purification by column chromatography on SiO2 and recrystallization was 82%.
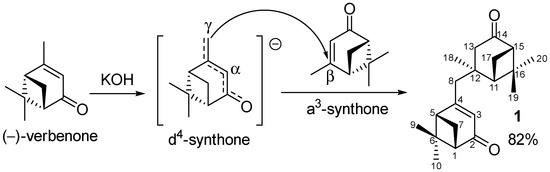
Scheme 1.
γ, β-Dimerization of (−)-verbenone.
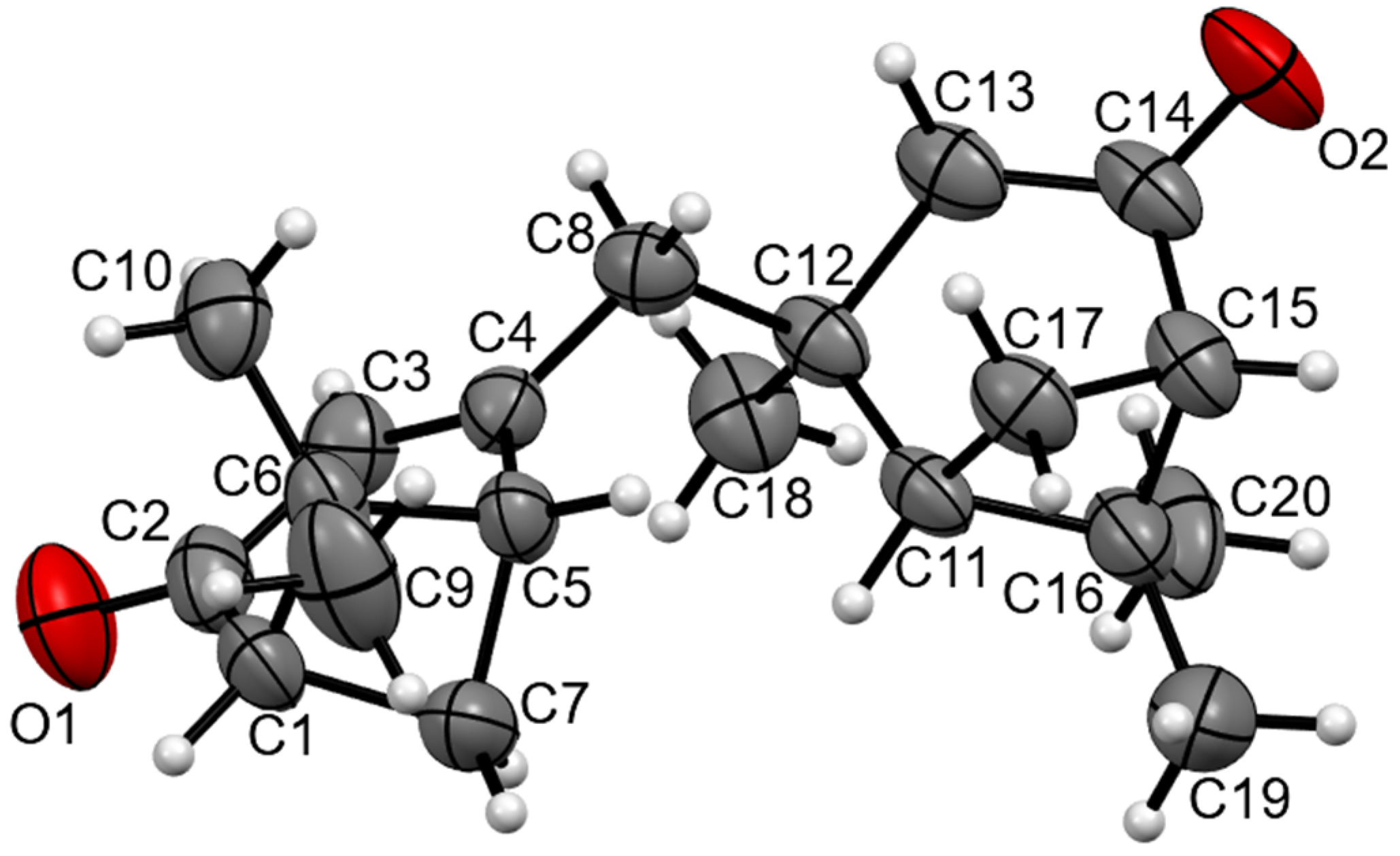
The molecular structure of the compound is illustrated in Figure 1 according to the X-ray diffraction data. The bond lengths and bond angles are the same as the statistical means [17]. In the crystal packing, the short H19A...O2 2.58 Å contact leads to the formation of 1D infinite chains of molecules.
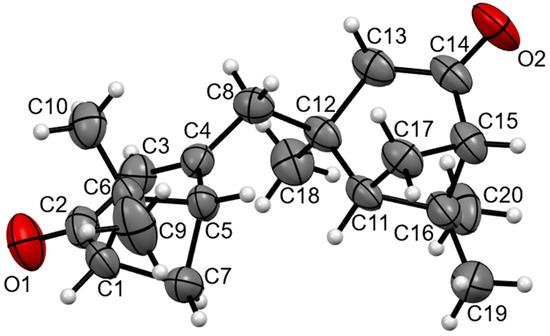
Figure 1.
The molecular structure of the title compound. Displacement ellipsoids are shown at a 50% probability level.
3. Materials and Methods
3.1. General
All reagents and solvents are commercially available and used as supplied. NMR spectra Spectral and analytical measurements were obtained at the Multi-Access Chemical Research Center SB RAS (Moscow, Russia) for spectral and analytical measurements. NMR spectra were registered on Bruker AV-600 spectrometer (resonance frequency for 1H 600.3 MHz) (Moscow, Russia) Chemical shifts for 1H and 13C were measured relative to the internal standard CDCl3 (δ 7.24 ppm for 1H, 76.9 ppm for 13C). For structure determination and NMR signal assignment in 1 2D correlation spectra, 1H-1H (COSY, NOESY) and 1H-13C (HSQC, HMBC) were used. Optical rotation: polAAr 3005 spectrometer, CHCl3 solution. HR-MS: DFS-Thermo-Scientific spectrometer (Thermo Fisher Scientific, Moscow, Russia) in a full scan mode (15–500 m/z, 70eVelectron-impact ionization, direct sample introduction).
3.2. Synthesis of (1S,5R)-6,6-Dimethyl-4-(((1S,2S,5S)-2,6,6-trimethyl-4-oxobicyclo[3.1.1]heptan-2-yl)methyl)bicyclo[3.1.1]hept-3-en-2-one
KOH (2.85 g, 50.8 mmol, 7.29 eq) was ground in a mortar, (−)-verbenone (1.047 g, 6.97 mmol, −210.5 (c 0.77, CHCl3), SAFC) was added, mixed thoroughly and left for a week at RT. Then, H2O (8 mL) was added, the reaction mixture was extracted with Et2O (3 × 10 mL), and the combined organic layers were dried over Na2SO4. The desiccant was filtered off, the solvent was distilled off, the residue was purified by column chromatography on SiO2 with EtOAc/hexane gradient (0–100%), and product was recrystallized from the Et2O/hexane solvent. The compound 1 (856 mg, 2.85 mmol, 82%) was obtained. −1.1 (c 0.15, CHCl3), mp 118.8 °C (with decompozition). 1H-NMR (CDCl3, δH): 0.99 (s, 3H, H-10), 1.00 (s, 3H, H-20), 1.19 (s, 3H, H-18), 1.34 (s, 3H, H-19), 1.47 (s, 3H, H-9), 1.68 (d; 1H, 2J 10.4 Hz, H-17anti), 1.89 (dd, 1H, 6.0, 5.2 Hz, H-11), 2.05 (d; 1H, 2J 9.2 Hz, H-7anti), 2.31–2.38 (m, 3H, H-5, H-8, H-8′), 2.32 (d, 1H, 2J 19.8 Hz, H-13), 2.42 (d, 1H, 2J 19.8 Hz, H-13′), 2.50–2.56 (m, 2H, H-17sin, H-15), 2.64 (td, 1H, 5.8, 1.8 Hz, H-1), 2.80 (dt, 1H, 9.2, 5.6 Hz, H-7sin), 5.67–5.69 (m, 1H, H-3). 13C-NMR, δC: 57.37 (d; C-1), 203.31 (s; C-2), 124.43 (d; C-3), 169.05 (s; C-4), 50.33 (d; C-5), 53.86 (s; C-6), 41.09 (t; C-7), 50.48 (t; C-8), 26.61 (q; C-9), 22.32 (q; C-10), 51.85 (d; C-11), 36.47 (s; C-12), 47.84 (t; C-13), 212.71 (s; C-14), 57.55 (d; C-15), 40.75 (s; C-16), 25.21 (t; C-17), 26.23 (q; C-18), 27.38 (q; C-19), 25.79 (q; C-20). HR-MS: 300.2083 ([M+], C20H28O2; calcd 300.2084).Copies of 1H-NMR, 13C-NMR, 2D correlation spectra 1H-1H (COSY, NOESY) and 1H-13C (HSQC, HMBC) and mass spectra of 1 are presented in Supplementary Materials.
3.3. Crystallography Details
X-ray crystallography study of the crystals were carried out on a Bruker Kappa Apex II CCD diffractometer using φ,ω-scans of narrow (0.5°) frames with Mo Kα radiation (λ = 0.71073 Å) and a graphite monochromator. The structure was solved by direct methods using the SHELXT-2014/5 [18] and was refined by the full-matrix least-squares method against all F2 in anisotropic approximation using the SHELXL-2018/3 [18]. The hydrogen atom positions were calculated with the riding model. Absorption corrections were applied using the empirical multi-scan method with the SADABS program [19]. The compound was monoclinic, space group P21/c, a = 11.499(1), b = 12.488(1), c = 13.095(1) Å, β = 111.706(3)°, V = 1747.2(3) Å3, Z = 4, C20H28O2, Dc = 1.142 r/cm3, μ = 0.072 mm−1, F(000) = 656, crystal size 0.90 × 0.20 × 0.08 mm3, independent reflections 3448, wR2 = 0.1873, S = 1.01 for all reflections (R = 0.0604 for 2313 I > 2σ). The obtained crystal structures were analyzed for short contacts between non-bonded atoms using the PLATON program [20,21]. Tables listing detailed crystallographic data, atomic positional parameters, and bond lengths and angles are available as CCDC 2209697 from the Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
4. Conclusions
The interaction of verbenone with KOH without solvent led to the Michael addition of verbenone-extended enolate and provided its γ,β-dimer with an 82% preparative yield.
Supplementary Materials
The following data are available online. 1H-NMR, 13C-NMR, 2D correlation spectra 1H-1H (COSY, NOESY) and 1H-13C (HSQC, HMBC) and mass spectra of 1.
Author Contributions
O.V.A. performed the chemical synthesis. The registration and interpretation of the NMR data and structure characterization of one were made by D.V.K. X-ray crystallography study of the crystals was carried out by I.Y.B. The manuscript was written by O.V.A., K.P.V. and N.F.S. All authors have read and agreed to the published version of the manuscript.
Funding
The current work was financially supported by the Russian Foundation for Basic Research (Grant No 19-29-04011).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
Authors would like to acknowledge the Multi-Access Chemical Research Center SB RAS for spectral and analytical measurements.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ravid, U.; Putievsky, E.; Katzir, I.; Lewinsohn, E.; Dudai, N. Identification of (1R)(+)-verbenone in essential oils of Rosmarinus officinalis L. Flavour Fragr. J. 1997, 12, 109–112. [Google Scholar] [CrossRef]
- Sun, H.; Zhang, T.; Fan, Q.; Qi, X.; Zhang, F.; Fang, W.; Jiang, J.; Chen, F.; Chen, S. Identification of Floral Scent in Chrysanthemum Cultivars and Wild Relatives by Gas Chromatography-Mass Spectrometry. Molecules 2015, 20, 5346–5359. [Google Scholar] [CrossRef] [PubMed]
- Lindgren, B.S.; Miller, D.R. Effect of Verbenone on Five Species of Bark Beetles (Coleoptera: Scolytidae) in Lodgepole Pine Forests. Environ. Entomol. 2002, 31, 759–765. [Google Scholar] [CrossRef]
- Brand, J.M.; Bracke, J.W.; Britton, L.N.; Markovetz, A.J.; Barras, S.J. Bark beetle pheromones: Production of verbenone by a mycangial fungus of Dendroctonus frontalis. J. Chem. Ecol. 1976, 2, 195–199. [Google Scholar] [CrossRef]
- Rauchdia, M.; Alib, M.A.; Roucouxa, A.; Denicourt-Nowickia, A. Novel access to verbenone via ruthenium nanoparticles-catalyzed oxidation of α-pinene in neat water. Appl. Catal. A Gen. 2018, 550, 266–273. [Google Scholar] [CrossRef]
- EMurphy, F.; Mallat, T.; Baiker, A. Allylic oxofunctionalization of cyclic olefins with homogeneous and heterogeneous catalysts. Catal. Today 2000, 57, 115–126. [Google Scholar] [CrossRef]
- Naser, B.A.; Al-Wabel, A.; Shams, S.; Ahamad, A.; Khan, S.A.; Anwar, F. Essential oils used in aromatherapy: A systemic review. Asian Pac. J. Trop. Biomed. 2015, 5, 601–611. [Google Scholar]
- Ardashov, O.V.; Pavlova, A.V.; Il’ina, I.V.; Morozova, E.A.; Korchagina, D.V.; Karpova, E.V.; Volcho, K.P.; Tolstikova, T.G.; Salakhutdinov, N.F. Highly potent activity of (1R,2R,6S)-3-methyl-6-(prop-1-en-2-yl)cyclohex-3-ene-1,2-diol in animal models of Parkinson’s disease. J. Med. Chem. 2011, 54, 3866–3874. [Google Scholar] [CrossRef]
- Winkler, J.D.; Bhattacharya, S.K.; Liotta, F.; Batey, R.A.; Heffernan, G.D.; Cladingboel, D.E.; Kelly, R.C. Stereoselective synthesis of a synthon for the A-ring of taxol from R-(+)- verbenone. Tetrahedron Lett. 1995, 36, 2211–2214. [Google Scholar] [CrossRef]
- Zhou, X.-H.; Davies, J.; Huang, S.; Luo, J.; Shi, Z.; Polishak, B.; Cheng, Y.-J.; Kim, T.-D.; Johnson, L.; Jen, A. Facile structure and property tuning through alteration of ring structures in conformationally locked phenyltetraene nonlinear optical chromophores. J. Mater. Chem. 2011, 221, 4437–4444. [Google Scholar] [CrossRef]
- Ju, C.; Song, S.; Hwang, S.; Kim, C.; Kim, M.; Gu, J.; Oh, Y.-K.; Lee, K.; Kwon, J.; Lee, K.; et al. Discovery of novel (1S)-(-)-verbenone derivatives with anti-oxidant and anti-ischemic effects. Bioorganic Med. Chem. Lett. 2013, 23, 5421–5425. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Li, Z.; Wu, J.; Jiang, Z.; Luo, J.; Jen, A.K.-Y. Design, synthesis, and properties of nonlinear optical chromophores based on a verbenone bridge with a novel dendritic acceptor. J. Mater. Chem. C 2018, 6, 2840–2847. [Google Scholar] [CrossRef]
- Bessière-Chrétien, Y.; el Gaïed, M.M. Réduction, par le lithium dissous dans l’ammoniac liquide, de cétones α-cyclopropaniques dérivés de terpènes. Bull. Soc. Chim. Fr. 1971, 6, 2189–2194. [Google Scholar]
- Kato, M.; Watanabe, M.; Vogler, B.; Awen, B.Z.; Masuda, Y.; Tooyama, Y.; Yoshikoshi, A. The Use of 4,4-disubstituted nopinones for natural-product synthesis. synthesis of elemanoid sesquiterpenes. J. Org. Chem. 1991, 56, 7071–7076. [Google Scholar] [CrossRef]
- Ballester, P.; Costa, A.; Raso, A.G.; Gómez-Solivellas, A. Dienediolates from unsaturated carboxylic acids: Michael addition of dilithium buta-l,3-diene-l,I -diolate (from crotonic acid) to unsaturated ketones. J. Chem. Soc. Perkin Trans. I 1988, 1711–1717. [Google Scholar] [CrossRef]
- Paquette, L.A.; Bzowej, E.I.; Branan, B.M.; Stanton, K.J. Oxidative coupling of the enolate anion of (1R)-(+)-verbenone with Fe(III) and Cu(II) salts. Two modes of conjoining this bicyclic ketone across a benzene ring. J. Org. Chem. 1995, 60, 7277–7283. [Google Scholar] [CrossRef]
- Allen, F.H.; Kenard, O.; Watson, D.G.; Bramer, L.; Orpen, A.G.; Taylor, R.J. Tables of bond lengths determined by X-ray and neutron diffraction. Part 1. Bond lengths in organic compounds. J. Chem. Soc. Perkin Trans. II 1987, 2, S1–S19. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Cryst. 2015, C71, 3–8. [Google Scholar]
- SADABS; Version 2008-1; Bruker AXS: Madison, WI, USA, 2008.
- Spek, A.L. PLATON, A Multipurpose Crystallographic Tool; (Version 10M); Utrecht University: Utrecht, The Netherlands, 2003. [Google Scholar]
- Spek, A.L. Single-crystal structure validation with the program PLATON. J. Appl. Crystallogr. 2003, 36, 7–13. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).