Assembly of Sn(IV)-Porphyrin Cation Exhibiting Supramolecular Interactions of Anion···Anion and Anion···π Systems
Abstract
1. Introduction
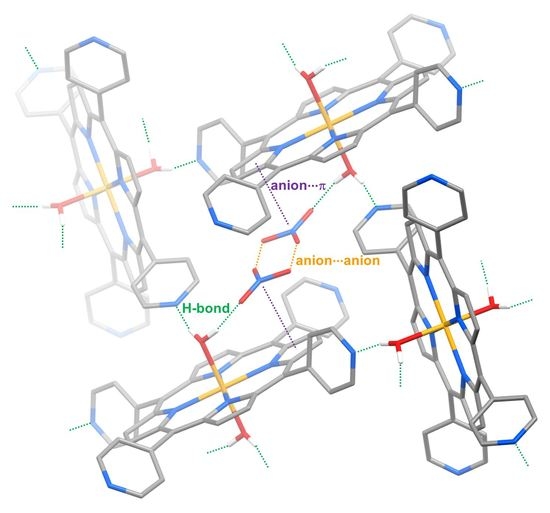
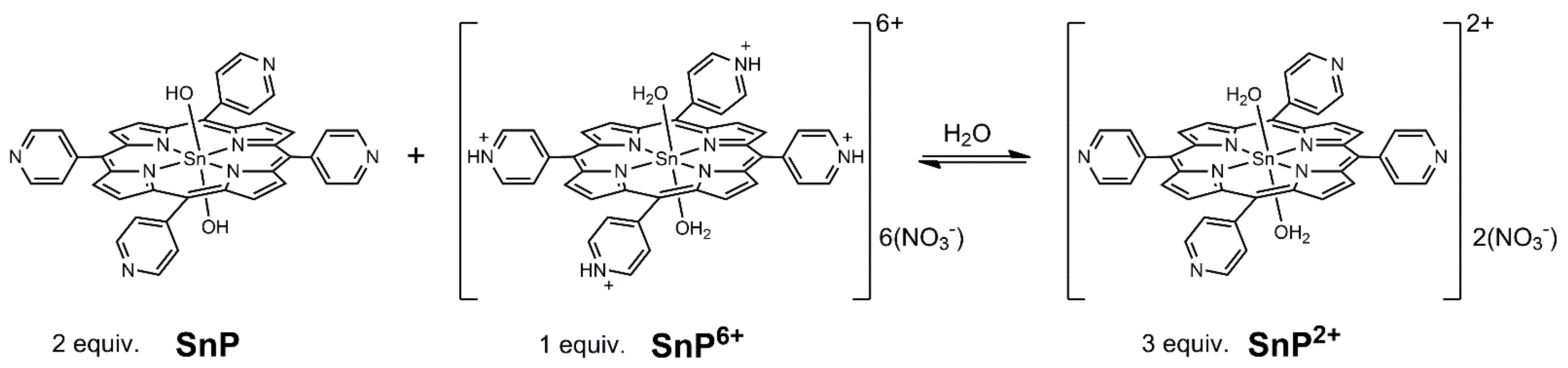
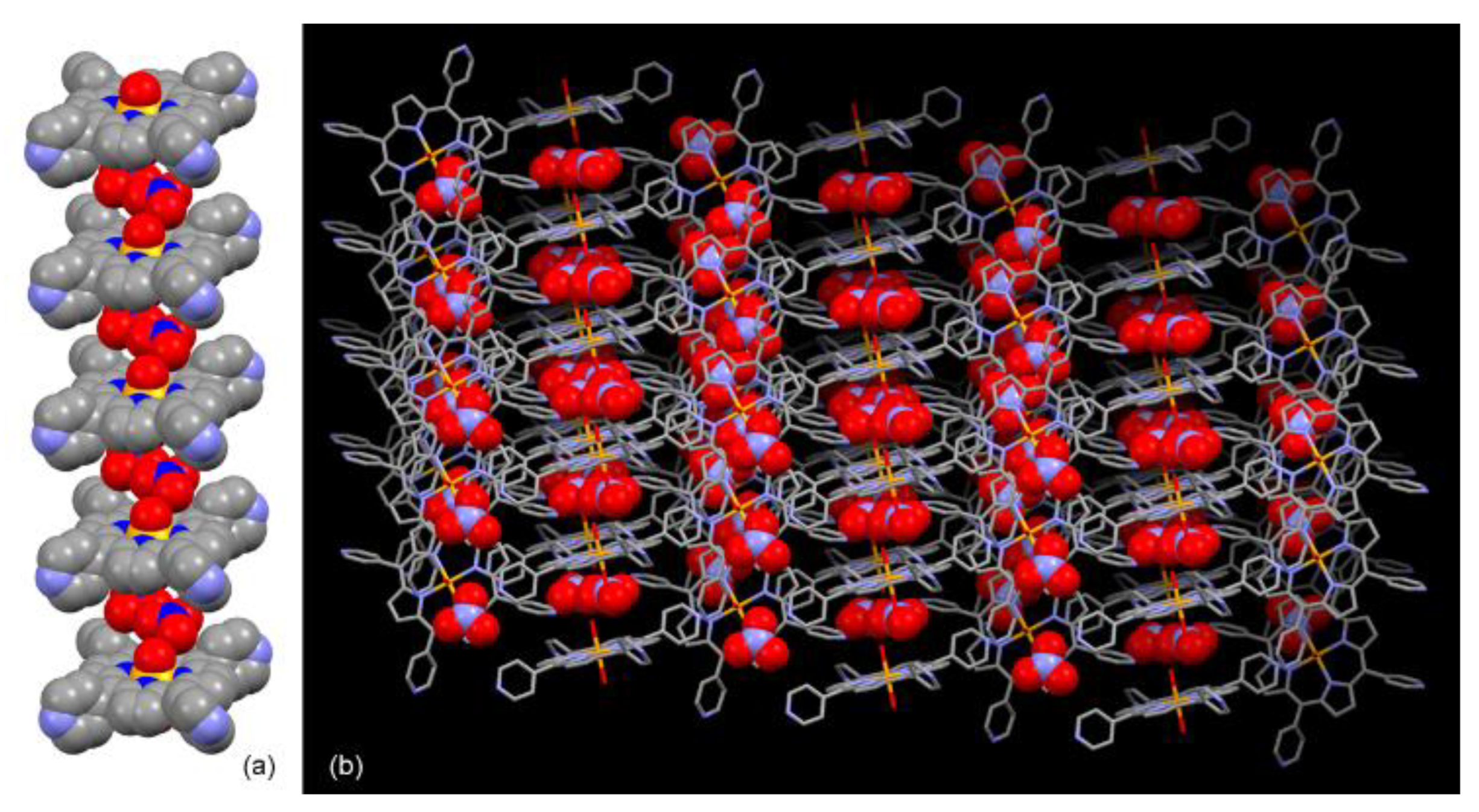
2. Results and Discussion
3. Materials and Methods
3.1. Preparation of Trans-diaqua[meso-tetrakis(4-pyridyl)porphyrinato]Sn(IV) Dinitrate
3.2. X-ray Crystal Structure Determination
4. Conclusions
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Drain, C.M.; Hupp, J.T.; Suslick, K.S.; Wasielewski, M.R.; Chen, X. A perspective on four new porphyrin-based functional materials and devices. J. Porphyr. Phthalocyanines 2002, 6, 243–258. [Google Scholar] [CrossRef]
- Milic, T.N.; Chi, N.; Yablon, D.G.; Flynn, G.W.; Batteas, J.D.; Drain, C.M. Controlled hierarchical self-assembly and deposition of nanoscale photonic materials. Angew. Chem. Int. Ed. 2002, 41, 2117–2119. [Google Scholar] [CrossRef]
- Suslick, K.S.; Rakow, N.A.; Kosal, M.E.; Chou, J.-H. The materials chemistry of porphyrins and metalloporphyrins. J. Porphyr. Phthalocyanines 2000, 4, 407–413. [Google Scholar] [CrossRef]
- Chambron, J.-C.; Heitz, V.; Sauvage, J.-P. The Porphyrin Handbook; Kadish, K.M., Smith, K.M., Guilard, R., Eds.; Academic Press: San Diego, CA, USA, 2000; Volume 6, pp. 1–42. [Google Scholar]
- Chou, J.-H.; Nalwa, H.S.; Kosal, M.E.; Rakow, N.A.; Suslick, K.S. Applications of Porphyrins and Metalloporphyrins to Materials Chemistry. In The Porphyrin Handbook; Kadish, K.M., Smith, K.M., Guilard, R., Eds.; Academic Press: San Diego, CA, 2000; Volume 6, pp. 43–131. [Google Scholar]
- George, S.; Goldberg, I. Self-Assembly of Supramolecular Porphyrin Arrays by Hydrogen Bonding: New Structures and Reflections. Cryst. Growth Des. 2006, 6, 755–762. [Google Scholar] [CrossRef]
- Vinodu, M.; Goldberg, I. New assembly modes of porphyrin-based networks. CrystEngComm 2003, 5, 204–207. [Google Scholar] [CrossRef]
- Goldberg, I. Crystal engineering of porphyrin framework solids. Chem. Commun. 2005, 1243–1254. [Google Scholar] [CrossRef]
- Hasobe, T. Photo-and electro-functional self-assembled architectures of porphyrins. Phys. Chem. Chem. Phys. 2012, 14, 15975–15987. [Google Scholar] [CrossRef]
- Northrop, B.H.; Zheng, Y.-R.; Chi, K.-W.; Stang, P.J. Self-Organization in Coordination-Driven Self-Assembly. Acc. Chem. Res. 2009, 42, 1554–1563. [Google Scholar] [CrossRef]
- Horike, S.; Shimomura, S.; Kitagawa, S. Soft porous crystals. Nat. Chem. 2009, 1, 695–704. [Google Scholar] [CrossRef]
- Farha, O.K.; Shultz, A.M.; Sarjeant, A.A.; Nguyen, S.T.; Hupp, J.T. Active-Site-Accessible, Porphyrinic Metal-Organic Framework Materials. J. Am. Chem. Soc. 2011, 133, 5652–5655. [Google Scholar] [CrossRef]
- Lee, C.Y.; Farha, O.K.; Hong, B.J.; Sarjeant, A.A.; Nguyen, S.T.; Hupp, J.T. Light-Harvesting Metal-Organic Frameworks (MOFs): Efficient Strut-to-Strut Energy Transfer in Bodipy and Porphyrin-Based MOFs. J. Am. Chem. Soc. 2011, 133, 15858–15861. [Google Scholar] [CrossRef]
- Würthner, F.; Kaiser, T.E.; Saha-Möller, C.R. J-Aggregates: From Serendipitous Discovery to Supramolecular Engineering of Functional Dye Materials. Angew. Chem. Int. Ed. 2011, 50, 3376–3410. [Google Scholar] [CrossRef]
- Madueno, R.; Raisanen, M.T.; Silien, C.; Buck, M. Functionalizing hydrogen-bonded surface networks with self-assembled monolayers. Nature 2008, 454, 618–621. [Google Scholar] [CrossRef]
- Watson, M.D.; Fechtenkotter, A.; Müllen, K. Big Is Beautiful-“Aromaticity” Revisited from the Viewpoint of Macromolecular and Supramolecular Benzene Chemistry. Chem. Rev. 2001, 101, 1267–1300. [Google Scholar] [CrossRef]
- Hunter, C.A.; Sanders, J.K.M. The nature of .Pi.-.Pi. Interactions. J. Am. Chem. Soc. 1990, 112, 5525–5534. [Google Scholar] [CrossRef]
- Zang, L.; Che, Y.; Moore, J.S. One-Dimensional Self-Assembly of Planar π-Conjugated Molecules: Adaptable Building Blocks for Organic Nanodevices. Acc. Chem. Res. 2008, 41, 1596–1608. [Google Scholar] [CrossRef]
- Medforth, C.J.; Wang, Z.; Martin, K.E.; Song, Y.; Jacobsen, J.L.; Shelnutt, J.A. Self-assembled porphyrin nanostructures. Chem. Commun. 2009, 7261–7277. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-J.; Jo, H.J.; Kim, J.; Kim, S.-Y.; Kim, D.; Kim, K. Supramolecular self-assembly of tin(IV) porphyrin channels stabilizing single-file chains of water molecules. CrystEngComm 2005, 7, 417–420. [Google Scholar] [CrossRef]
- Shee, N.K.; Lee, C.-J.; Kim, H.-J. Hexacoordinated Sn(IV) porphyrin-based square-grid frameworks exhibiting selective uptake of CO2 over N2. Bull. Korean Chem. Soc. 2022, 43, 103–109. [Google Scholar] [CrossRef]
- Shee, N.K.; Jo, H.J.; Kim, H.-J. Coordination framework materials fabricated by the self-assembly of Sn(IV) porphyrins with Ag(I) ions for the photocatalytic degradation of organic dyes in wastewater. Inorg. Chem. Front. 2022, 9, 1270–1280. [Google Scholar] [CrossRef]
- Arnold, D.P.; Blok, J. The coordination chemistry of tin porphyrin complexes. Coord. Chem. Rev. 2004, 248, 299–319. [Google Scholar] [CrossRef]
- Sanders, J.K.M.; Bampos, N.; Clyde-Watson, Z.; Darling, S.L.; Hawley, J.C.; Kim, H.-J.; Mak, C.C.; Webb, S.J. The Porphyrin Handbook; Kadish, K.M., Smith, K.M., Guilard, R., Eds.; Academic Press: San Diego, CA, USA, 2000; Volume 3, pp. 1–48. [Google Scholar]
- Kim, H.-J.; Bampos, N.; Sanders, J.K.M. Assembly of Dynamic Heterometallic Oligoporphyrins Using Cooperative Zinc-Nitrogen, Ruthenium-Nitrogen, and Tin-Oxygen Coordination. J. Am. Chem. Soc. 1999, 121, 8120–8121. [Google Scholar] [CrossRef]
- Kim, H.J.; Park, K.-M.; Ahn, T.K.; Kim, S.K.; Kim, K.S.; Kim, D.; Kim, H.-J. Novel fullerene–porphyrin–fullerene triad linked by metal axial coordination: Synthesis, X-ray crystal structure, and spectroscopic characterizations of trans-bis([60]fullerenoacetato)tin(iv) porphyrin. Chem. Commun. 2004, 22, 2594–2595. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Jeon, W.S.; Lim, J.H.; Hong, C.S.; Kim, H.-J. Synthesis, X-ray crystal structure, and electrochemistry of trans-bis(ferrocenecarboxylato)(tetraphenylporphyrinato)tin(IV). Polyhedron 2007, 26, 2517–2522. [Google Scholar] [CrossRef]
- Kim, H.J.; Jang, J.H.; Choi, H.; Lee, T.; Ko, J.; Yoon, M.; Kim, H.-J. Photoregulated Fluorescence Switching in Axially Coordinated Tin(IV) Porphyrinic Dithienylethene. Inorg. Chem. 2008, 47, 2411–2415. [Google Scholar] [CrossRef]
- Kim, S.H.; Kim, H.; Kim, K.; Kim, H.-J. The first tin(IV) porphyrin complex with chiral amino acid ligands: Synthesis, characterization and X-ray crystal structure of trans-bis(L-prolinato)[5,10,15,20-tetrakis-(4-pyridyl)porphyrinato]tin(IV). J. Porphyrins Phthalocyanines 2009, 13, 805–810. [Google Scholar] [CrossRef]
- Singh, A.P.; Park, B.B.; Kim, H.-J. Facile C-C bond cleavage of β-diketones by tin(IV) porphyrin complex. Tetrahedron Lett. 2012, 53, 6456–6459. [Google Scholar] [CrossRef]
- Kim, H.J.; Shee, N.K.; Park, K.-M.; Kim, H.-J. Assembly and X-ray crystal structures of heterometallic multiporphyrins with complementary coordination between ruthenium (II) and tin (IV) porphyrins. Inorg. Chim. Acta 2019, 488, 1–7. [Google Scholar] [CrossRef]
- Shee, N.K.; Kim, M.K.; Kim, H.-J. Fluorescent chemosensing for aromatic compounds by supramolecular complex composed of tin(IV) porphyrin, viologen, and cucurbit[8]uril. Chem. Commun. 2019, 55, 10575–10578. [Google Scholar] [CrossRef]
- Kim, M.K.; Shee, N.K.; Lee, J.; Yoon, M.; Kim, H.-J. Photoinduced Electron Transfer upon Supramolecular Complexation of (Porphyrinato) Sn-Viologen with Cucurbit [7] uril. Photochem. Photobiol. Sci. 2019, 18, 1996–2002. [Google Scholar] [CrossRef]
- Shee, N.K.; Kim, M.K.; Kim, H.-J. Supramolecular Porphyrin Nanostructures Based on Coordination-Driven Self-Assembly and Their Visible Light Catalytic Degradation of Methylene Blue Dye. Nanomaterials 2020, 10, 2314. [Google Scholar] [CrossRef] [PubMed]
- Shee, N.K.; Kim, H.-J. Self-Assembled Nanomaterials Based on Complementary Sn(IV) and Zn(II)-Porphyrins, and Their Photocatalytic Degradation for Rhodamine B Dye. Molecules 2021, 26, 3598. [Google Scholar] [CrossRef] [PubMed]
- Shee, N.K. Kim, H.-J. Three Isomeric Zn(II)-Sn(IV)-Zn(II) Porphyrin-Triad-Based Supramolecular Nanoarchitectures for the Morphology-Dependent Photocatalytic Degradation of Methyl Orange. ACS Omega 2022, 7, 9775–9784. [Google Scholar] [CrossRef] [PubMed]
- Jang, J.H.; Jeon, K.-S.; Oh, S.; Kim, H.-J.; Asahi, T.; Masuhara, H.; Yoon, M. Synthesis of Sn-porphyrin-intercalated trititanate nanofibers: Optoelectronic properties and photocatalytic activities. Chem. Mater. 2007, 19, 1984–1991. [Google Scholar] [CrossRef]
- Kim, W.; Park, J.; Jo, H.J.; Kim, H.-J.; Choi, W. Visible light photocatalysts based on homogeneous and heterogenized tin porphyrins. J. Phys. Chem. C 2008, 112, 491–499. [Google Scholar] [CrossRef]
- Kim, W.; Tachikawa, T.; Majima, T.; Li, C.; Kim, H.-J.; Choi, W. Tin-porphyrin sensitized TiO2 for the production of H2 under visible light. Energy Environ. Sci. 2010, 3, 1789–1795. [Google Scholar] [CrossRef]
- Kim, H.; Kim, W.; Mackeyev, Y.; Lee, G.-S.; Kim, H.-J.; Tachikawa, T.; Hong, S.; Lee, S.; Kim, J.; Wilson, L.J.; et al. Selective oxidative degradation of organic pollutants by singlet oxygen-mediated photosensitization: Tin porphyrin versus C60 aminofullerene systems. Environ. Sci. Tech. 2012, 46, 9606–9613. [Google Scholar] [CrossRef]
- Li, C.; Park, K.-M.; Kim, H.-J. Ionic assembled hybrid nanoparticle consisting of tin(IV) porphyrin cations and polyoxomolybdate anions, and photocatalytic hydrogen production by its visible light sensitization. Inorg. Chem. Commun. 2015, 60, 8–11. [Google Scholar] [CrossRef]
- Faul, C.F.J.; Antonietti, M. Ionic self-assembly: Facile synthesis of supramolecular materials. Adv. Mater. 2003, 15, 673–683. [Google Scholar] [CrossRef]
- He, Q.; Tu, P.; Sessler, J.L. Supramolecular Chemistry of Anionic Dimers, Trimers, Tetramers and Clusters. Chem. 2018, 4, 46–93. [Google Scholar] [CrossRef]
- Nelyubina, Y.V.; Antipin, M.Y.; Lyssenko, K.A. Anion–anion interactions: Their nature, energy and role in crystal formation. Russ. Chem. Rev. 2010, 79, 167–187. [Google Scholar] [CrossRef]
- Dance, I.; Scudder, M. Supramolecular Motifs: Concerted Multiple Phenyl Embraces between Ph4P+ Cations are Attractive and Ubiquitous. Chem.-Eur. J. 1996, 2, 481–486. [Google Scholar] [CrossRef] [PubMed]
- Chesman, A.S.R.; Hodgson, J.L.; Izgorodina, E.I.; Urbatsch, A.; Turner, D.R.; Deacon, G.B.; Batten, S.R. Anion-anion interactions in the crystal packing of functionalized methanide anions: An experimental and computational study. Cryst. Growth Des. 2014, 14, 1922–1932. [Google Scholar] [CrossRef]
- Wysokiński, R.; Zierkiewicz, W.; Michalczyk, M.; Scheiner, S. Crystallographic and theoretical evidences of anion···anion interaction. ChemPhysChem 2021, 22, 818–821. [Google Scholar] [CrossRef]
- Nelyubina, Y.V.; Lyssenko, K.A.; Golovanov, D.G.; Antipin, M.Y. NO3−···NO3− and NO3−···π interactions in the crystal of urea nitrate. CrystEngComm 2007, 9, 991–996. [Google Scholar] [CrossRef]
- Khan, S.; Roy, S.; Harms, K.; Bauzá, A.; Frontera, A.; Chattopadhyay, S. Observation of an anion···anion interaction in a square planar copper(II) Schiff base complex: DFT study and CSD analysis. Inorg. Chim. Acta 2019, 487, 465–472. [Google Scholar] [CrossRef]
- Dougherty, D.A. Cation-pi interactions in chemistry and biology: A new view of benzene, Phe, Tyr, and Trp. Science 1996, 271, 163–168. [Google Scholar] [CrossRef]
- Ma, J.C.; Dougherty, D.A. The Cation-π Interaction. Chem. Rev. 1997, 97, 1303–1324. [Google Scholar] [CrossRef]
- Sussman, J.L.; Harel, M.; Frolow, F.; Oefner, C.; Goldman, A.; Tolker, L.; Silman, I. Atomic structure of acetylcholinesterase from Torpedo californica: A prototypic acetylcholine-binding protein. Science 1991, 253, 872–879. [Google Scholar] [CrossRef]
- Quiñonero, D.; Garau, C.; Rotger, C.; Frontera, A.; Ballester, P.; Costa, A.; Deyà, P.M. Anion-π Interactions: Do They Exist? Angew. Chem. 2002, 114, 3539–3542. [Google Scholar] [CrossRef]
- Schottel, B.L.; Chifotides, H.T.; Dunbar, K.R. Anion-π Interactions. Chem. Soc. Rev. 2008, 37, 68–83. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.-X.; Wang, M.-X. Exploring Anion-π Interactions and Their Applications in Supramolecular Chemistry. Acc. Chem. Res. 2020, 53, 1364–1380. [Google Scholar] [CrossRef] [PubMed]
- Jo, H.J.; Jung, S.H.; Kim, H.-J. Synthesis and Hydrogen-Bonded Supramolecular Assembly of trans-Dihydroxotin(IV) Tetrapyridylporphyrin Complexes. Bull. Korean Chem. Soc. 2004, 25, 1869–1873. [Google Scholar]
- Jo, H.J.; Kim, S.H.; Kim, H.-J. Supramolecular Assembly of Tin(IV) Porphyrin Cations Stabilized by Ionic Hydrogen-Bonding Interactions. Bull. Korean Chem. Soc. 2015, 36, 2348–2351. [Google Scholar] [CrossRef]
- Tan, X.-W.; Li, H.-F.; Li, C.-H. Syntheses, Crystal Structures and Fluorescence Properties of Two Complexes Constructed from Rigid 1,4-bis(4-methyl-imidazolyl)Benzene. Chin. J. Inorg. Chem. 2017, 33, 156–162. [Google Scholar]
- Ni, T.-J.; Yuan, Q.-T.; Zhang, W.; Yang, Z.-J.; Yu, H.-H.; Cai, H.-R. Influence of Solvents on the Formation of Silver(I) Complexes with the Flexible 1,3,5-Tris(triazol-1-ylmethyl)-2,4,6-trimethylbenzene. Chin. J. Inorg. Chem. 2017, 33, 2177–2185. [Google Scholar]
- Bar, A.K.; Raghothoma, S.; Moon, D.; Mukherjee, P.S. Three-Component Self-Assembly of a Series of Triply Interlocked Pd12 Coordination Prisms and Their Non-Interlocked Pd6 Analogues. Chem. Eur. J. 2012, 18, 3199–3209. [Google Scholar] [CrossRef]
- Area Detector Control and Integration Software, Version 6.22; Bruker AXS Inc.: Madison, WI, USA, 1996.
- Sheldrick, G.M. SADABS: Empirical Absorption and Correction Software; University of Gottingen: Gottingen, Germany, 1999. [Google Scholar]
- Program for Solution and Refinement of Crystal Structures, Version 6.10; Bruker AXS Inc.: Madison, WI, USA, 2000.
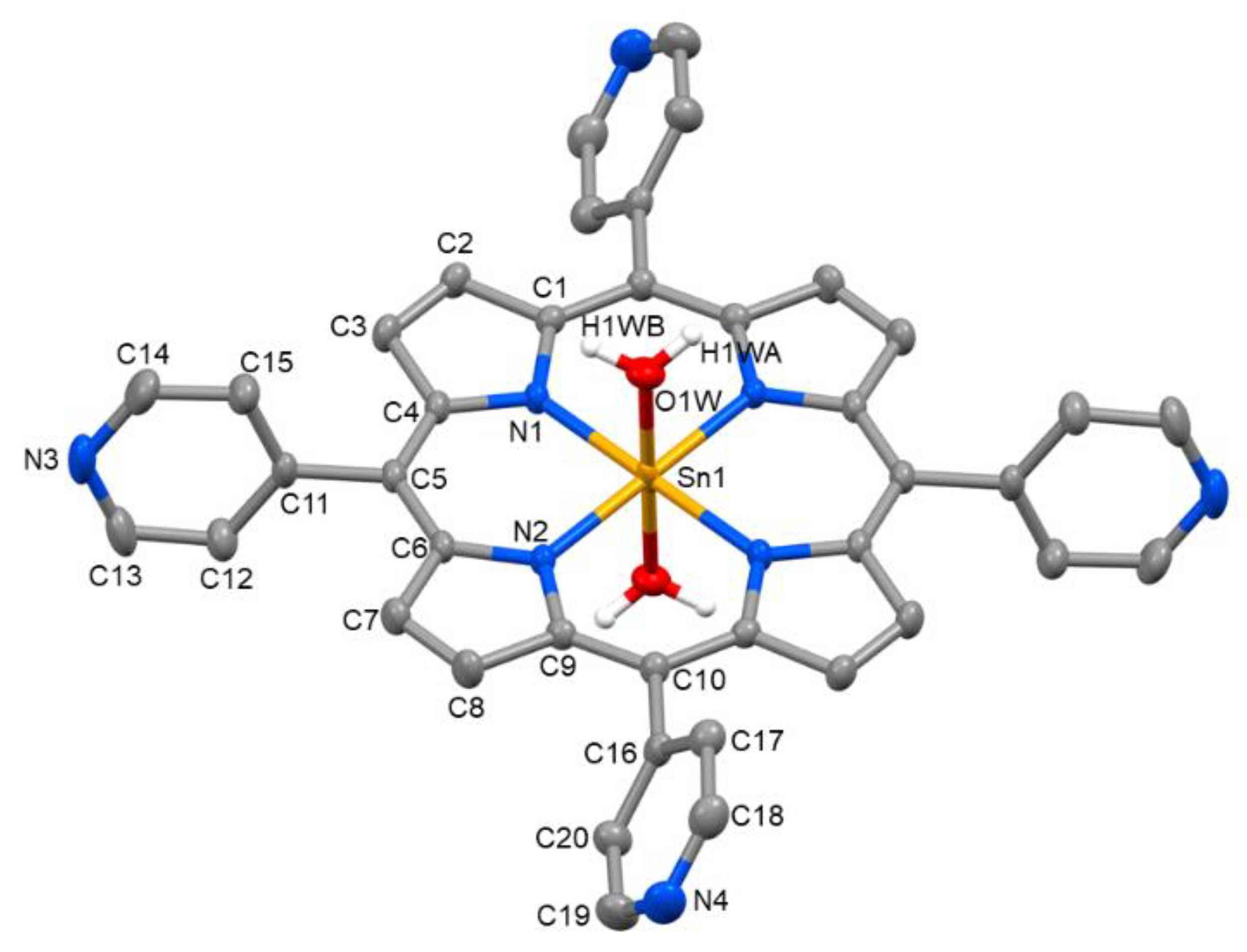



| SnP2+ | SnP6+ [a] | SnP [b] | |
|---|---|---|---|
| Sn–O1W | 2.093(2) | 2.1024(19) | 2.014(3) |
| Sn–N1 | 2.093(2) | 2.087(2) | 2.098(3) |
| Sn–N2 | 2.089(2) | 2.078(2) | 2.104(3) |
| ∠N1–Sn–N2 | 90.27(9) | 90.52(8) | 90.19(11) |
| ∠N1–Sn–O1W | 90.95(9) | 89.64(8) | 93.12(11) |
| ∠N2–Sn–O1W | 90.43(9) | 87.56(8) | 89.83(12) |
| D–H···A | d(D–H) | d(H···A) | d(D···A) | ∠(DHA) |
|---|---|---|---|---|
| O1W–H1WA···N3i | 0.82 | 1.90 | 2.695(4) | 165.2 |
| O1W–H1WB···O2 | 0.82 | 1.81 | 2.610(7) | 167.6 |
| O1W–H1WB···N5 | 0.82 | 2.62 | 3.394(7) | 157.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, H.-J. Assembly of Sn(IV)-Porphyrin Cation Exhibiting Supramolecular Interactions of Anion···Anion and Anion···π Systems. Molbank 2022, 2022, M1454. https://doi.org/10.3390/M1454
Kim H-J. Assembly of Sn(IV)-Porphyrin Cation Exhibiting Supramolecular Interactions of Anion···Anion and Anion···π Systems. Molbank. 2022; 2022(4):M1454. https://doi.org/10.3390/M1454
Chicago/Turabian StyleKim, Hee-Joon. 2022. "Assembly of Sn(IV)-Porphyrin Cation Exhibiting Supramolecular Interactions of Anion···Anion and Anion···π Systems" Molbank 2022, no. 4: M1454. https://doi.org/10.3390/M1454
APA StyleKim, H.-J. (2022). Assembly of Sn(IV)-Porphyrin Cation Exhibiting Supramolecular Interactions of Anion···Anion and Anion···π Systems. Molbank, 2022(4), M1454. https://doi.org/10.3390/M1454