Abstract
The crystal structure and vibrational spectra of the tetraethylammonium salt of tribromotriphylphosphinonickel(II) is reported. Br-Ni-Br angles vary between 108° and 121°, deviating from perfect tetrahedral angles.
1. Introduction
Four-coordinate, approximately tetrahedral complexes of nickel(II) are widely used to illustrate magnetic and spectroscopic properties of these typical first-row compounds with the d8 electron configuration [1,2]. Mixed ligand spheres are of particular interest and have given rise to detailed and illustrative analyses of different types of bonding and magnetic properties involving the highly degenerate electronic ground state [1,2]. Structural data and vibrational spectra are essential bases for such analyses. The structure of the tribromotriphenylphosphinenickel(II) anion, [NiBr3(PPh3)]− has been reported with the [Ph4As]+ counterion [3]. The structure of a trichloro analog was published in 2001 [4], decades later than [3], and with the tetraethylammonium counterion. We report here the structure of tetraethylammonium tribromo(triphenylphosphine)nickelate(II) in order to document structures for trichloro and tribromo anionic complexes with identical counterions and to determine deviations of Br-Ni-Br and Cl-Ni-Cl angles from those for perfect tetrahedral coordination. In addition, we report infrared (IR) and Raman spectra of the solid title compound (see Supplementary Materials), to complement the reported magnetic moments and electronic absorption data for the title compound and related four-coordinate nickel(II) complexes [5].
2. Results
The four-coordinate nickel(II) center in this structure shows approximately tetrahedral coordination geometry, as illustrated in Figure 1. The bromo ligands are disordered, also illustrated in the Figure.
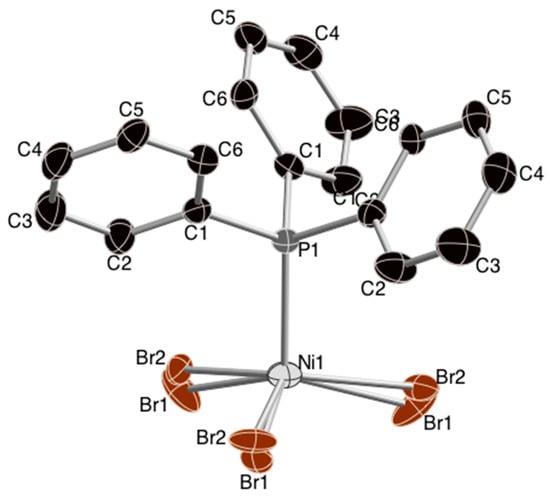
Figure 1.
Structure of the [NiBr3(PPh3)]− anion with atom numbering. Hydrogen atoms are omitted for clarity. Ellipsoids for non-hydrogen atoms are shown at the 30% level.
The alkyl chains of the tetraethylammonium counterion are disordered, as reported for the chloro analog [4]. The X-Ni-X angles from the title compound, [NiBr3(PPh3)][Ph4As] [3] and [NiCl3(PPh3)][Et4N] [4] are summarized in Table 1.

Table 1.
Br-Ni-Br and Cl-Ni-Cl angles for [NiBr3(PPh3)]− and [NiCl3(PPh3)]−.
The title compound shows Br-Ni-Br angles very similar to those published previously [3], wider than for perfect tetrahedral coordination by up to 8°. In contrast, the chloro analog [4] shows angles wider by 5° than for perfect tetrahedral structures. These deviations are relatively small and do not significantly affect the ligand-field electronic structure, where the most significant effects occur due to the differences in Ni-phosphine and Ni-halide bonding [1,2]. Full structural data are available at CCDC, deposition number 2166023.
The Raman and IR spectra of the crystalline title compound are shown in Figure 2. Peak positions are given in Table 2. Several, but not all vibrational transitions are observed both in IR and Raman spectra, as expected in perfect tetrahedral coordination geometry, showing therefore the close relationship of the title complex with idealized tetrahedral coordination geometry, despite the lower site symmetry for the nickel(II) ion in the title compound. Examples for such corresponding frequencies are the most intense Raman band at 3050 cm−1 and the corresponding IR peak at 3054 cm−1, identical values within the precision and spectral resolution of the instruments used.
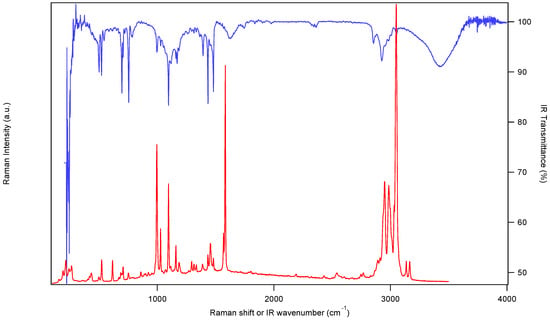
Figure 2.
IR (blue, top trace) and Raman (red, bottom trace) spectra of (solid NEt4)[NiBr3(PPh3)] recorded at room temperature.

Table 2.
Observed peak positions in the IR and Raman spectra. All values in cm−1 units.
3. Materials and Methods
(NEt4)NiBr3(PPh3) was crystallized from acetonitrile solutions of equivalent amounts of (NEt4)Br and PPh3 by adding a solution of anhydrous NiBr2 dissolved in warm acetonitrile. We did not use reflux conditions, in contrast to the synthesis described in [6]. The solution was stirred for 1h, then filtered. Dark green crystals were obtained from solutions in a desiccator. Quantitative reaction yields were not determined. Crystals can be handled in the air briefly for spectroscopic measurements. The Raman spectrum was measured on a Renishaw Invia Raman microscope. The IR spectrum was recorded with a Bio-Rad Excalibur FTIR spectrometer with a CsI beamsplitter.
Supplementary Materials
The structure is available as CCDC 2166023, deposited 10 April 2022. The material can be obtained free of charge via www.ccdc.cam.ac.uk/data_request/cif (accessed on 7 May 2022).
Author Contributions
F.B.-R.: synthesis, X-ray diffraction, spectroscopic measurements N.B.-D.: structure analysis. C.R.: writing, figure with spectroscopic data. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Natural Sciences and Engineering Research Council (Canada).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data for this study is available in this paper.
Acknowledgments
The authors thank Frank Schaper (Université de Montréal) for his invaluable help in the refinement of the structure, in particular with the disorder.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Davies, J.E.; Gerloch, M.; Phillips, D.J. Phosphine π-acceptor properties in dihalogenobis(triphenylphosphine)-nickel(II) and -cobalt(II). J. Chem. Soc. Dalton Trans. 1979, 1836–1842. [Google Scholar] [CrossRef]
- Gerloch, M.; Hanton, L.R. Donor and acceptor properties of triphenylphosphine ligands in trigonally distorted tetrahedral NiPX3− chromophores (X = bromine, iodine). Inorg. Chem. 1981, 20, 1046–1050. [Google Scholar] [CrossRef]
- Hanton, L.R.; Raithby, P.R. Tetraphenylarsonium Tribromo(triphenylphosphine)nickelate(II). Acta Cryst. 1980, 36, 2417–2419. [Google Scholar] [CrossRef]
- Smith, M.C.; Davies, S.C.; Hughes, D.L.; Evans, D.J. Tetraethylammonium trichloro(triphenylphosphine)nickelate(II). Acta Cryst. 2001, 57, m509–m510. [Google Scholar]
- Sacconi, L.; Mani, F.; Bencini, A. Chapter 50 Nickel. In Comprehensive Coordination Chemistry, 1st ed.; Wilkinson, G., Gillard, R., McCleverty, J., Eds.; Pergamon Press: Oxford, UK; New York, NY, USA, 1987; Volume 5, p. 62. [Google Scholar]
- Allender, G.; Smith, H.D. Five-Coordinate Ni(II) Complexes of 1,2-Bis(diphenylphosphino) o-carborane. Inorg. Chim. Acta 1978, 26, L38. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).