Abstract
Dimethyl sulfoxide (DMSO) is a cheap polar aprotic solvent used in organic synthesis and in pharmacology because of its low cost, high stability, and non-toxicity. Multicomponent reactions (MCRs) are highly convergent processes and have good atom, step, and pot economies. In this communication, the multicomponent transformation of salicylaldehyde, malononitrile dimer, and nitromethane in DMSO at room temperature was investigated to give 2,4-diamino-5-(nitromethyl)-5H-chromeno[2,3-b]pyridine-3-carbonitrile in good yield. The structure of the earlier unknown compound was confirmed by means of elemental analysis, mass-, nuclear magnetic resonance, and infrared spectroscopy.
1. Introduction
Sustainable trends in chemistry are gaining momentum [1]. Organic chemistry is becoming “greener”, it reduces waste and uses more effective approaches. Dimethyl sulfoxide (DMSO) meets modern challenges in organic chemistry as it has aprotic properties, it is eco-friendly and it simplifies the treatment of a reaction mixture. Thus, it may be one of the most effective solvents today.
Multicomponent reactions (MCRs) are highly convergent processes that include two or more chemical reactions. These processes are often related to the PASE principles—atom, step, and pot economies [2]. Such a convergence leads to the formation of several bonds at once and, thus, to high bond-forming index (BFI) of the whole process [3]. Apparently, the mentioned advantages of MCRs in corporation with advantages of DMSO as a solvent are supposed to be even more effective, eco-friendly and useful for the development of new synthetic approaches [4]. In the literature, there is not a very large variety of multicomponent reactions in DMSO. In general, these are reactions in the iodine–DMSO system [5,6,7].
Chromeno[2,3-b]pyridines are an interesting class of three-fused heterocycles with broad medicinal, biological and industrial importance. Depending on their structure, these compounds show different kinds of pharmacological activity, such as antimicrobial [8], anticancer [9], antimyopic [10], neuroprotective [11], and other properties. In addition, 5-S-substituted chromeno[2,3-b]pyridines can inhibit the corrosion of mild steel [12]. At present, the best known are two anti-inflammatory commercial drugs: amlexanox and pranoprofen (Figure 1). Thus, the multicomponent design of new chromeno[2,3-b]pyridines is an important goal for organic and medicinal chemistry.
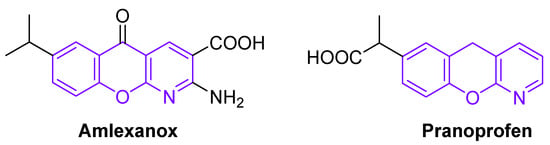
Figure 1.
Some biologically active chromeno[2,3-b]pyridines.
In the synthesis of different types of chromeno[2,3-b]pyridines, both multistep classical and multicomponent methods [13] are used. We have already published different multicomponent transformations leading to 5-C- and 5-P-substituted chromeno[2,3-b]pyridines [14,15,16,17,18].
2. Results and Discussion
We previously realized multicomponent reactions of salicylaldehydes, 2-aminoprop-1-ene-1,1,3-tricarbonitrile and malonic acid or dimethyl malonate into 2-(2,4-diamino-3-cyano-5H-chromeno[2,3-b]pyridin-5-yl)malonic acids or dimethyl 2-(2,4-diamino-3-cyano-5H-chromeno[2,3-b]pyridin-5-yl)malonate [19,20] (Scheme 1). These reactions were the first examples of a multicomponent synthesis of chromeno[2,3-b]pyrnidines in DMSO.
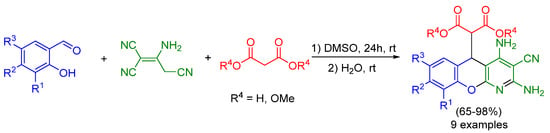
Scheme 1.
Reaction of salicylaldehyde, malononitrile dimer, and malonic acid or dimethyl malonate.
The application of this technique made it possible to obtain previously inaccessible heterocyclic compounds. Therefore, we decided to try to synthesize other chromeno[2,3-b]pyridines that were previously inaccessible to us under these conditions.
Now, we wish to report our results on the efficient multicomponent transformation of salicylaldehyde 1, 2-aminoprop-1-ene-1,1,3-tricarbonitrile 2, and nitromethane 3 into the novel 2,4-diamino-5-(nitromethyl)-5H-chromeno[2,3-b]pyridine-3-carbonitrile 4 in DMSO at room temperature (23 °C) for 24 h, as shown in Scheme 2.
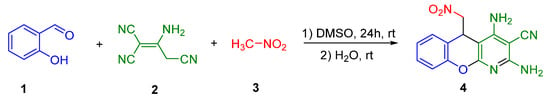
Scheme 2.
Reaction of salicylaldehyde 1, malononitrile dimer 2, and nitromethane 3.
When the reaction in DMSO was completed, water was added to the reaction mixture and the final chromeno[2,3-b]pyridine 4 was crystallized in pure form. Compound 4 was synthesized in 73% yield.
We also carried out the transformation described above in other aprotic solvents and catalyst–solvent systems developed by us (Table 1).

Table 1.
Optimization of multicomponent reaction conditions 1.
In aprotic polar solvents, the reaction proceeds with good yields (Table 1, Entries 1–3). The use of systems developed earlier by us did not give such good results (Table 1, Entries 4–9). In most cases, the target was not fixed (Table 1, Entries 4, 5, 7, and 8). In two cases (Table 1, Entries 6 and 9), there were very small yields of the compound, calculated from the 1H-NMR spectra. The best conditions were confirmed by our research (Table 1, Entry 1).
The BFI (bond-forming index) of this transformation was four since four new bonds were formed in one stage, namely 2 C–C bonds, 1 C–N, and 1 C–O bonds.
The structure of novel chromeno[2,3-b]pyridine 4 was confirmed by 1H, 13C NMR, and IR spectroscopy data, mass spectrometry data and elemental analysis (see Supplementary Materials). Only one set of signals was recorded in 1H and 13C-NMR spectra.
In 2017, 12-nitromethyl-12H-chromeno[2,3-c]isoquinolin-5-amines were synthesized from salicylaldehydes, homophthalonitrile and nitromethane using one-pot transformation in 20–92% yields [21]. The process proceeds in two stages without the isolation of intermediate compounds under the action of microwave radiation.
Earlier in our scientific group, 2-amino-4-(1-nitroalkyl)-4H-chromenes were synthesized from salicylaldehydes, nitroalkanes and malononitrile derivatives [22,23].
Taking into consideration our previous results and 1H-NMR monitoring data [19,24], the following mechanism for the multicomponent reaction of salicylaldehyde 1, 2-aminoprop-1-ene-1,1,3-tricarbonitrile 2, and nitromethane 3 was suggested, as shown in Scheme 3.
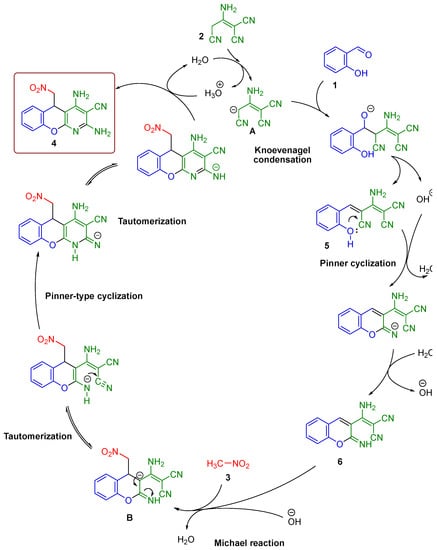
Scheme 3.
Mechanism of salicylaldehyde 1, malononitrile dimer 2, and nitromethane 3 transformation into chromeno[2,3-b]pyridine 4. Catalytic cycles are simplified.
The first stage of the process was a rapid formation of Knoevenagel adduct 5 with the expulsion of a hydroxide anion [25]. This hydroxide anion instantly catalyzed a rapid Pinner cyclization of adduct 5 into intermediate 6. Then, the Michael addition of nitromethane 3 occurred to form anion B. Next, there were successive tautomerizations and Pinner-type cyclization to the final 2,4-diamino-5-(nitromethyl)-5H-chromeno[2,3-b]-pyridine-3-carbonitrile 4.
3. Materials and Methods
3.1. General Methods
The solvents and reagents were purchased from commercial sources and used as received. 2-Aminoprop-1-ene-1,1,3-tricarbonitrile 2 (malononitrile dimer) was obtained from malononitrile according to the literature [26].
The melting point was measured with Gallenkamp melting-point apparatus (Gallenkamp & Co., Ltd., London, UK). 1H and 13C-NMR spectra were recorded in DMSO-d6 temperature. IR spectrum was registered with a Bruker ALPHA-T FT-IR spectrometer (Bruker Corporation, Billerica, MA, USA) in KBr pellets. The MS spectrum (EI = 70 eV) was obtained directly with a Kratos MS-30 spectrometer (Kratos Analytical Ltd., Manchester, UK). For elemental analysis, a 2400 Elemental Analyzer (Perkin Elmer Inc., Waltham, MA, USA) was used.
3.2. Multicomponent Synthesis of 2,4-Diamino-5-(nitromethyl)-5H-chromeno[2,3-b]pyridine-3-carbonitrile 4
Salicylaldehyde 1 (0.122 g, 1 mmol), 2-aminoprop-1-ene-1,1,3-tricarbonitrile 2 (0.132 g, 1 mmol) and nitromethane 3 (0.061 g, 1 mmol) were stirred in 5 mL of DMSO for 24 h at ambient temperature. After the reaction was completed, 15 mL of water was added to the solution. The formed solid was filtered, washed with well-chilled ethanol (3 mL × 2), and dried to isolate pure 2,4-diamino-5-(nitromethyl)-5H-chromeno[2,3-b]pyridine-3-carbonitrile 4.
2,4-Diamino-5-(nitromethyl)-5H-chromeno[2,3-b]pyridine-3-carbonitrile (4): Yellowish solid; yield—73% (0.217 g); mp = 271–272 °C (decomp.) (from DMSO-H2O); FTIR (KBr) cm−1: 3466 (NH2), 3337 (NH2), 3247 (NH2), 3153 (NH2), 2205 (CN), 1637 (C–C Ar), 1600 (C–C Ar), 1568 (C–C Ar), 1229 (NO2). 1H-NMR (300 MHz, DMSO-d6): δ 4.68 (d, 3J = 4.8 Hz, 1H, CH2), 4.86 (t, 3J = 4.8 Hz, 1H, CH), 6.52 (s, 2H, NH2), 6.78 (s, 2H, NH2), 7.07 (d, 3J = 8.0 Hz, 1H, CH Ar), 7.16 (t, 3J = 8.0 Hz, 1H, CH Ar), 7.24–7.36 (m, 2H, 2 CH Ar) ppm; 13C-NMR (75 MHz, DMSO-d6): δ 32.6 (C(5)H), 70.7 (C(3)-CN), 79.3 (CH2), 85.1 (C(4a)), 116.4 (C(9)H Ar), 120.3 (CN), 124.0 (2C, C(5a) and C(7)H Ar), 128.4 (C(8)H Ar), 128.9 (C(6)H Ar), 151.2 (C(9a)), 156.7 (C(2)-NH2), 159.9 (C(4)-NH2), 160.1 (C(1a)) ppm; MS (m/z, relative intensity %): 297 [M]+ (3), 250 [M – NO2 – H]+ (3), 237 [M – CH2NO2]+ (100), 77 [C6H5]+ (5); Anal. calcd. for C14H11N5O3: C, 56.57; H, 3.73; N, 23.56%; found: C, 56.65; H, 3.77; N, 23.47%.
4. Conclusions
The title compound, 2,4-diamino-5-(nitromethyl)-5H-chromeno[2,3-b]pyridine-3-carbonitrile, was synthesized in good yield using the mild and efficient multicomponent method with simple implementation and equipment and available starting materials. The new synthesized compound was characterized by spectroscopic methods (NMR, IR and MS-EI), and elemental analysis.
Supplementary Materials
The following are available online, compound 4 spectra: Figure S1. 1H-NMR spectrum of 2,4-diamino-5-(nitromethyl)-5H-chromeno[2,3-b]pyridine-3-carbonitrile 4 in DMSO-d6; Figure S2. 13C-NMR spectrum of 2,4-diamino-5-(nitromethyl)-5H-chromeno[2,3-b]pyridine-3-carbonitrile 4 in DMSO-d6; Figure S3. MS (EI) spectrum of 2,4-diamino-5-(nitromethyl)-5H-chromeno[2,3-b]pyridine-3-carbonitrile 4; Figure S4. IR spectrum of 2,4-diamino-5-(nitromethyl)-5H-chromeno[2,3-b]pyridine-3-carbonitrile 4.
Author Contributions
Conceptualization, F.V.R. and M.N.E.; methodology, Y.E.R. and M.N.E.; investigation, Y.E.R. and O.I.M.; writing—original draft preparation, Y.E.R. and F.V.R.; writing—review and editing, M.N.E.; supervision, M.N.E. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data for the compound presented in this study are available in the Supplementary Materials of this article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Welton, T. Solvents and sustainable chemistry. Proc. Math. Phys. Eng. Sci. 2015, 471, 20150502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, W.; Yi, W.-B. Pot, Atom, and Step Economy (PASE) Synthesis; Chapter: Multicomponent Reactions (MCRs); Springer: Cham, Switzerland, 2019; pp. 27–40. [Google Scholar] [CrossRef] [Green Version]
- Domling, A.; Wang, W.; Wang, K. Chemistry and biology of multicomponent reactions. Chem. Rev. 2012, 112, 3083–3135. [Google Scholar] [CrossRef] [Green Version]
- Brahmachari, G. Green synthetic approaches for biologically relevant heterocycles: Advanced synthetic techniques—An overview. In Green Synthetic Approaches for Biologically Relevant Heterocycles, Volume 1: Advanced Synthetic Techniques, 2nd ed.; Brahmachari, G., Ed.; Elsevier Science Publishing Company, Inc.: Amsterdam, The Netherlands, 2021; Chapter 1; pp. 1–8. [Google Scholar] [CrossRef]
- Gao, Q.; Liu, S.; Wu, X.; Wu, A.-X. Povarov-Type Reaction Using Methyl as New Input: Direct Synthesis of Substituted Quinolines by I2-Mediated Formal [3 + 2 + 1] Cycloaddition. Org. Lett. 2014, 16, 4582–4585. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wu, X.; Gao, Q.; Geng, X.; Zhao, P.; Wu, Y.-D.; Wu, A.-X. Diamination/Oxidative Cross-Coupling/Bicyclization of Anilines and Methyl Ketones: Direct I2-Promoted Synthesis of 1,2-Fused Oxindoles. Org. Lett. 2017, 19, 408–411. [Google Scholar] [CrossRef] [PubMed]
- Jana, A.; Bhaumick, P.; Panday, A.K.; Mishra, R.; Choudhury, L.H. I2/DMSO mediated multicomponent reaction for the synthesis of 2-arylbenzo[d]imidazo[2,1-b]thiazole derivatives. Org. Biomol. Chem. 2019, 17, 5316–5330. [Google Scholar] [CrossRef]
- Ghoneim, A.A.; El-Farargy, A.F.; Abdelaziz, S. Synthesis and Antimicrobial Activities of New S-Nucleosides of Chromeno[2,3-b]Pyridine Derivatives and C-Nucleosides of [1,2,4]Triazolo[1,5-a]Quinoline Derivatives. Nucleosides Nucleotides Nucleic Acids 2014, 33, 583–596. [Google Scholar] [CrossRef]
- Oliveira-Pinto, S.; Pontes, O.; Lopes, D.; Sampaio-Marques, B.; Costa, M.D.; Carvalho, L.; Gonçalves, C.S.; Costa, B.M.; Maciel, P.; Ludovico, P.; et al. Unravelling the anticancer potential of functionalized chromeno[2,3-b]pyridines for breast cancer treatment. Bioorg. Chem. 2020, 100, 103942. [Google Scholar] [CrossRef]
- Ukawa, K.; Ishiguro, T.; Kuriki, H.; Nohara, A. Synthesis of the metabolites and degradation products of 2-amino-7-isopropyl-5-oxo-5H-(1)benzopyrano(2,3-b)pyridine-3-carboxylic acid (Amoxanox). Chem. Pharm. Bull. 1985, 33, 4432–4437. [Google Scholar] [CrossRef] [Green Version]
- Oset-Gasque, M.J.; González, M.P.; Pérez-Peña, J.; García-Font, N. Toxicological and pharmacological evaluation, antioxidant, ADMET and molecular modeling of selected racemic chromenotacrines {11-amino-12-aryl-8,9,10,12-tetrahydro-7H-chromeno[2,3-b]quinolin-3-ols} for the potential prevention and treatment of Alzheimer’s disease. Eur. J. Med. Chem. 2014, 74, 491–501. [Google Scholar] [CrossRef] [Green Version]
- Verma, C.; Olasunkanmi, L.O.; Obot, I.B.; Ebenso, E.E.; Quraishi, M.A. 2,4-Diamino-5-(phenylthio)-5H-chromeno [2,3-b]pyridine-3-carbonitriles as green and effective corrosion inhibitors: Gravimetric, electrochemical, surface morphology and theoretical studies. RSC Adv. 2016, 6, 53933–53948. [Google Scholar] [CrossRef]
- Elinson, M.N.; Ryzhkova, Y.E.; Ryzhkov, F.V. Multicomponent design of chromeno[2,3-b]pyridine systems. Russ. Chem. Rev. 2021, 90, 94–115. [Google Scholar] [CrossRef]
- Vereshchagin, A.N.; Elinson, M.N.; Anisina, Y.E.; Ryzhkov, F.V.; Goloveshkin, A.S.; Bushmarinov, I.S.; Zlotin, S.G.; Egorov, M.P. Pot, atom and step economic (PASE) synthesis of 5-isoxazolyl-5H-chromeno[2,3-b]pyridine scaffold. Mendeleev Commun. 2015, 25, 424–426. [Google Scholar] [CrossRef]
- Vereshchagin, A.N.; Elinson, M.N.; Anisina, Y.E.; Ryzhkov, F.V.; Goloveshkin, A.S.; Novikov, R.A.; Egorov, M.P. Synthesis, structural, spectroscopic and docking studies of new 5C-substituted 2,4-diamino-5H-chromeno[2,3-b]pyridine-3-carbonitriles. J. Mol. Struct. 2017, 1146, 766–772. [Google Scholar] [CrossRef]
- Elinson, M.N.; Vereshchagin, A.N.; Anisina, Y.E.; Fakhrutdinov, A.N.; Goloveshkin, A.S.; Egorov, M.P. Pot-, Atom- and Step-Economic (PASE) Multicomponent approach to the 5-(Dialkylphosphonate)-Substituted 2,4-Diamino-5H-chromeno[2,3-b]pyridine scaffold. Eur. J. Org. Chem. 2019, 2019, 4171–4178. [Google Scholar] [CrossRef]
- Elinson, M.N.; Vereshchagin, A.N.; Anisina, Y.E.; Egorov, M.P. Efficient Multicomponent Approach to the Medicinally Relevant 5-aryl-chromeno[2,3-b]pyridine Scaffold. Polycycl. Aromat. Compd. 2020, 40, 108–115. [Google Scholar] [CrossRef]
- Elinson, M.N.; Vereshchagin, A.N.; Anisina, Y.E.; Krymov, S.K.; Fakhrutdinov, A.N.; Egorov, M.P. Selective multicomponent ‘one-pot’ approach to the new 5-(4-hydroxy-6-methyl-2-oxo-2H-pyran-3-yl)chromeno[2,3-b]pyridine scaffold in pyridine–ethanol catalyst/solvent system. Monatsh. Chem. 2019, 150, 1073–1078. [Google Scholar] [CrossRef]
- Ryzhkova, Y.E.; Elinson, M.N.; Maslov, O.I.; Fakhrutdinov, A.N. Multicomponent Synthesis of 2-(2,4-Diamino-3-cyano-5H-chromeno[2,3-b]pyridin-5-yl)malonic Acids in DMSO. Molecules 2021, 26, 6839. [Google Scholar] [CrossRef] [PubMed]
- Ryzhkova, Y.E.; Maslov, O.I.; Elinson, M.N. Dimethyl 2-(2,4-Diamino-3-cyano-5H-chromeno[2,3-b]pyridin-5-yl)malonate. Molbank 2022, 2022, M1308. [Google Scholar] [CrossRef]
- Festa, A.A.; Storozhenko, O.A.; Ndoutoume, D.R.B.; Varlamov, A.V.; Voskressensky, L.G. Sequential three-component reaction of homophthalonitrile, salicylaldehydes and nitromethane. Mendeleev Commun. 2017, 27, 451–453. [Google Scholar] [CrossRef]
- Elinson, M.N.; Ilovaisky, A.I.; Merkulova, V.M.; Belyakov, P.A.; Chizhov, A.O.; Nikishin, G.I. Solvent-free cascade reaction: Direct multicomponent assembling of 2-amino-4H-chromene scaffold from salicylaldehyde, malononitrile or cyanoacetate and nitroalkanes. Tetrahedron 2010, 66, 4043–4048. [Google Scholar] [CrossRef]
- Elinson, M.N.; Dorofeev, A.S.; Miloserdov, F.M.; Ilovaisky, A.I.; Feducovich, S.K.; Belyakov, P.A.; Nikishin, G.I. Catalysis of Salicylaldehydes and Two Different C-H Acids with Electricity: First Example of an Efficient Multicomponent Approach to the Design of Functionalized Medicinally Privileged 2-Amino-4H-Chromene Scaffold. Adv. Synth. Catal. 2008, 350, 591–601. [Google Scholar] [CrossRef]
- Ryzhkov, F.V.; Ryzhkova, Y.E.; Elinson, M.N.; Vorobyev, S.V.; Fakhrutdinov, A.N.; Vereshchagin, A.N.; Egorov, M.P. Catalyst-Solvent System for PASE Approach to Hydroxyquinolinone-Substituted Chromeno[2,3-b]pyridines Its Quantum Chemical Study and Investigation of Reaction Mechanism. Molecules 2020, 25, 2573. [Google Scholar] [CrossRef] [PubMed]
- Patai, S.; Israeli, Y. 411. The kinetics and mechanisms of carbonyl–methylene condensations. Part VII. The reaction of malononitrile with aromatic aldehydes in ethanol. J. Chem. Soc. 1960, 2025–2030. [Google Scholar] [CrossRef]
- Mittelbach, M. An improved and facile synthesis of 2-amino-1,1,3-tricyanopropene. Mon. Chem. Chem. Mon. 1985, 116, 689–691. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).