Abstract
The X-ray structure of the title compound has been determined for the first time. Its molecular structure is in good agreement with those previously determined for similar benzyl halides and the angles between the ring–Cl bonds in adjacent molecules show values in good agreement with an early Zeeman quadrupole spectroscopy study.
1. Introduction
The isomerically pure 4-chlorobenzyl chloride 1 was first reported in 1878 [1] as being a low-melting solid, prepared by chlorination of pure 4-chlorotoluene. This was in contrast to the liquid product reported earlier from chlorination of toluene, which contained a significant proportion of 2-chlorobenzyl chloride. The compound is described as melting at 29 °C and being highly volatile, undergoing ready sublimation at normal temperatures [1]. Perhaps because of these properties, its X-ray structure has not so far been determined, although it is readily available from many major suppliers. However, there is an early report in which the 35Cl Zeeman quadrupole spectrum of a single crystal of 1 is used to gain some structural information [2]. We have now determined the structure of 1 and compare it both with X-ray structures of similar benzylic chlorides and the Zeeman quadrupole spectrum structural information.
2. Results
Crystals suitable for diffraction were obtained from the inner surface of a commercial bottle which had been stored for >5 years, allowing slow sublimation. The resulting structure (Figure 1) shows a perfectly planar chlorobenzene ring with the CH2–Cl bond almost orthogonal.
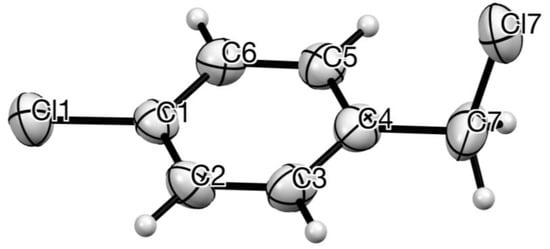
Figure 1.
Molecular structure of 1 with anisotropic displacement ellipsoids drawn at 50% probability level and showing numbering system used. Bond lengths and angles: C(1)–Cl(1) 1.724 (7), C(1)–C(2) 1.387 (9), C(2)–C(3) 1.385 (9), C(3)–C(4) 1.373 (9), C(4)–C(5) 1.382 (8), C(5)–C(6) 1.393 (9), C(6)–C(1) 1.378 (9), C(4)–C(7) 1.499 (10), C(7)–Cl(7) 1.791 (9) Å; C(4)–C(7)–Cl(7) 111.1 (5), C(4)–C(7)–Cl(7) plane to C(1)–C(2)–C(3)–C(4)–C(5)–C(6) mean plane 86.2°.
Relatively few simple benzyl halides have so far been characterised by X-ray diffraction (Figure 2), but these include both benzyl chloride 2 [3] and benzyl bromide 4 [3], which are liquids at room temperature and the hindered benzyl chloride 3 recently reported by us [4]. Rather remarkably, of the 48 possible 2-, 3- or 4-halobenzyl halides (halogen = F, Cl, Br, I), compound 1 appears to be only the third to have its X-ray structure determined, the previous two being 4-bromobenzyl bromide 5 [5] and 2-iodobenzyl bromide 6 [6]. The key structural parameters for these are compared in Table 1, and it is clear that there is a good level of consistency between the structure of 1 and the previously reported structures.
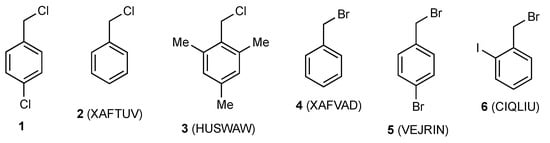
Figure 2.
Crystallographically characterised benzyl halides with CSD reference codes.

Table 1.
Geometric parameters for 1 and other simple benzyl halides.
In the unit cell of 1, there are four molecules, aligned as shown in Figure 3, with no significant intermolecular interactions. The early Zeeman quadrupole spectroscopy study was able to derive various structural parameters [2], but the one of most relevance here is the angle between the C–Cl bonds of different molecules in the crystal. The spectral quality was only good enough to evaluate this parameter for the ring C–Cl bonds, since the lower frequency signal associated with the CH2–Cl bonds was too weak. In addition, the author proposed a relative orientation that had two of the four molecules switched end for end compared to the arrangement shown in Figure 3, but did note this possibility which would not affect the key angles between C–Cl bonds in adjacent molecules.
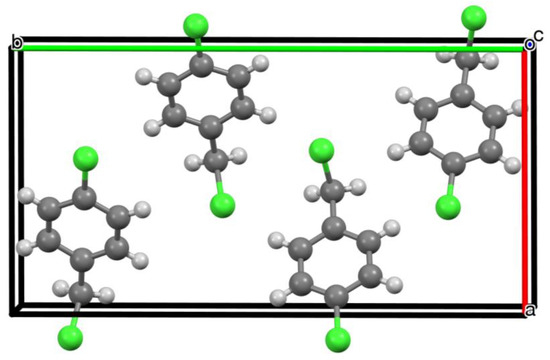
Figure 3.
Unit cell of 1 viewed along the c axis showing arrangement of the 4 molecules.
The paper concludes that in the crystal the angle between ring C–Cl bonds in different molecules is either 67 ± 1° or 8 ± 1°. Taking the planes defined by C(2), C(1), C(6) and Cl(1) (Figure 1) for each of the four molecules in the unit cell, our structure (Figure 3) gives three different values for this angle: 69.4° and 68.5° for the larger angle and 9.75° for the smaller one, in remarkable agreement with those derived from the Zeeman spectra over 60 years ago.
In summary, we obtained the X-ray crystal structure of 4-chlorobenzyl chloride for the first time and found the molecule to have a fully planar chlorobenzene ring with the CH2Cl bond essentially orthogonal to it. Bond lengths and angles are consistent with the few previous structures of benzyl halides, and the alignment of the ring to Cl bonds in adjacent molecules in the unit cell is in remarkably good agreement with that predicted in an early Zeeman quadrupole spectroscopy study.
3. Experimental Section
The structure was determined on a Rigaku SCX mini diffractometer using graphite monochromated Mo Kα radiation λ = 0.71075 Å.
Crystal data for C7H6Cl2, M = 161.03 g·mol–1, colourless prism, crystal dimensions 0.32 × 0.17 × 0.09 mm, orthorhombic, space group Pna21 (No. 33), a = 9.1724 (14), b = 17.714 (4), c =4.4440 (9) Å, α = β = γ = 90.00°, V = 722.1 (2) Å3, Z = 4, Dcalc = 1.481 g·cm–3, T = 173 K, R1 = 0.0734, Rw2 = 0.1874 for 1547 reflections with I > 2σ(I), and 82 variables, Rint 0.0839, goodness of fit on F2 0.983. Data have been deposited at the Cambridge Crystallographic Data Centre as CCDC 2087798 (see Supplementary Materials). The data can be obtained free of charge from the Cambridge Crystallographic Data Centre via http://www.ccdc.cam.ac.uk/getstructures. The structure was solved by direct methods and refined by full-matrix least-squares against F2 (SHELXL Version 2018/3 [7]).
Supplementary Materials
The following are available online, crystallographic data for 1 in cif format, check-cif file and molfile for 1.
Author Contributions
A.M.Z.S. collected the X-ray data and solved the structure; R.A.A. designed the study, analysed the data and wrote the paper. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Jackson, C.L.; Field, A.W. Über Parachlorobenzylchlorid und seine Derivate. Ber. Dtsch. Chem. Ges. 1878, 11, 904–905. [Google Scholar] [CrossRef] [Green Version]
- Meal, H.C. Zeeman quadrupole spectra of p-chloroaniline and p-chlorobenzylchloride. J. Chem. Phys. 1956, 24, 1011–1017. [Google Scholar] [CrossRef]
- Nayak, S.K.; Sathishkumar, R.; Guru Row, T.N. Directing role of functional groups in selective generation of C–H…π interactions: In situ cryo-crystallographic studies on benzyl derivatives. CrystEngComm 2010, 12, 3112–3118. [Google Scholar] [CrossRef]
- Aitken, R.A.; Slawin, A.M.Z. 2,4,6-Trimethylbenzyl chloride (α2-chloroisodurene). Molbank 2020, 2020, M1156. [Google Scholar] [CrossRef]
- Jones, P.G.; Kus, P.; Dix, I. X-Ray structure determinations of bromo- and/or bromomethyl-substituted benzenes: C–H…Br, C–Br…Br, and C–Br…π interactions. Z. Naturforsch. Teil B 2012, 67, 1273–1281. [Google Scholar] [CrossRef]
- Betz, R.; Klufers, P. 1-Bromomethyl-2-iodobenzene. Acta Crystallogr. Sect E 2007, 63, o4753. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A short history of SHELXL. Acta Crystallogr. Sect. A 2008, 64, 112–122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).