Abstract
Herein, we report the chromatography-free synthesis of 2-hydroxy-3-octyloxybenzaldehyde by the alkylation of 2,3-dihydroxybenzaldehyde as a promising precursor for new SalEn-type complexes with transition metals. The structure of the product is elucidated by means of 1H and 13C-NMR spectra, high-resolution mass spectrometry with electrospray ionization (ESI-HRMS) and Fourier-transform infrared spectroscopy (FTIR).
1. Introduction
Polymeric complexes of transition metals with SalEn-type ligands represent a family of materials with a wide range of potential applications in energy storage [1,2,3,4], (photo) electrocatalysis [5,6,7,8,9], and chemical and biological sensing [10,11,12]. One of the key benefits of the SalEn-type coordination materials is the ability to fine tune the functional properties by varying the substituents of the ligand. Long alkyl chains attached to the ligand of NiSalen polymer may decrease the phase transition temperature of this material, which may allow the development of temperature-sensitive materials with low trigger temperature and increased electrochemical stability. NiSalen complexes are usually synthesized from the 3-substituted salicylaldehyde; thus, 2-hydroxy-3-octyloxybenzaldehyde was considered as a precursor for the preparation of NiSalEn-bearing octyl chains [13,14,15,16]. In addition, the 2-hydroxy-3-octyloxybenzaldehyde is a promising material in the field of biomedicine, since alkyl-substituted salicylic acids are actively used as selective ligands of the cannabinoid CB2 receptor and are also an intermediate product of salviandic acid, which has a therapeutic effect in coronary heart disease [17,18,19].
In the framework of the development of thermoresistant and temperature-switchable conductive polymers, here, we present the synthesis and characterization of a new NiSalen precursor, 2-hydroxy-3-octyloxybenzaldehyde, obtained by direct alkylation of 2,3-dihydroxybenzaldehyde with octyl bromide for further targeted modification of polymer structures. The alkylation of 2,3-dihydroxybenzaldehyde proceeds with low selectivity, resulting in a mixture of mono- and bis-alkylated products, which are usually separated chromatographically. To avoid the time- and material-consuming chromatographic separation, we developed an alternative isolation route via the template approach with the complexation of the target product. Ni (II) was chosen as a templating ion for its known ability for the complexation of the salicylaldehydes and the low solubility of the resulting complexes. The structure of the product was elucidated by means of 1H and 13C-NMR spectroscopy, high-resolution mass spectrometry with electrospray ionization (ESI-HRMS) and Fourier-transform infrared spectroscopy (FTIR).
2. Results
In this study, 2-hydroxy-3-octyloxybenzaldehyde was obtained by alkylation of 2,3-dihydroxybenzaldehyde with octyl bromide using a modified method [17]. The synthetic route for the target compound is outlined in Scheme 1. The alkylation of 2,3-dihydroxybenzaldehyde affords a mixture of 3- and 2-alkylated isomers, 1 and 2, respectively, along with 2,3-dialkylated product 3, which are normally separated using flash chromatography. NMR yields of 1 and 3 in crude reaction mixture were found to be 18% and 21%, respectively (Figure S5). However, the separation of these products with close Rf values is quite difficult, especially for large loadings. To avoid the chromatographic separation, we proposed a chemical method for the isolation of product 1 based on differences in reactivity. Namely, only product 1 is able to form a stable complex with Ni2+ due to the presence of salicylaldehyde’s chelating functionality, while 2 and 3 are inert to this ion. Treatment of the reaction mixture with nickel acetate after simple workup led to the precipitation of the greenish-yellow Ni complex of 1. The free ligand 1 was then released from the isolated complex by hydrolysis with 20% aqueous H2SO4.
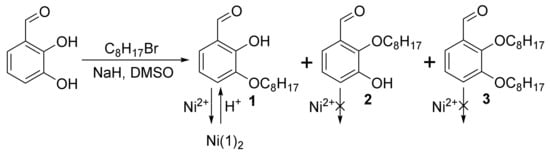
Scheme 1.
Synthesis of 1 and its isolation via the nickel complex.
The 1H-NMR spectrum of the product (Figure S1) shows a set of 2-hydroxy-3-octylbenzaldehyde-related signals: phenolic and aldehyde singlets at 10.99 and 9.92 ppm, respectively, a typical pattern of 1,2,3-trisubstituted aromatics with two doublets at 7.17 and 7.12 ppm, along with a triplet at 6.94 ppm. The alkoxy group chain is represented by a triplet at 4.05, attributed to the α-methylene group, a pseudo-triplet at 1.86, a large multiplet around 1.60–1.20 and a methyl triplet at 0.89.
The 13C-NMR spectrum (Figure S2) contains a complete set of signals: a carbonyl at 196.6, an aryl set at 152.1, 147.9, 124.7, 121.1, 119.7, 119.6 and eight signals from the octyloxy group at 69.7, 31.9, 29.5, 29.3, 29.3, 26.1, 22.8 and 14.2. The exact mass of the [M − H]− ion, determined by ESI-HRMS in negative mode (Figure S3), was found to be 249.1495 (249.1496 as calcd. for C15H21O3−). The FTIR spectrum recorded in KBr (Figure S4) contained a characteristic C = O vibration at 1659 cm−1.
Herein, we report the direct alkylation of 2,3-dihydroxybenzaldehyde with octyl bromide, which affords a valuable precursor for new SalEn-type complexes with transition metals. The resulting compound may be used further in electrocatalytic and photoelectrochemical reactions and also act as a promising material for creating energy storage devices.
3. Materials and Methods
3.1. General Considerations
All chemicals used in the synthesis were of “reagent-grade” purity and were purchased from local suppliers. Moreover, 2,3-dihydroxybenzaldehyde was sublimed twice at 1 Pa prior to use. DMSO was distilled over CaH2 and stored over 4 Å molecular sieves prior to use. The progression of the reaction was monitored by TLC, using SiO2 sheets with a fluorescent indicator F254. The Fourier-transform infrared spectra were recorded on a Shimadzu IRaffinity-1 FTIR spectrophotometer (Shimadzu Europa GmbH, Kyoto, Japan) in KBr pellets. The 1H and 13C-NMR spectra were acquired on a Bruker Avance 400 spectrometer (Bruker Analytische Messtechnik GmbH, Rheinstetten, Germany) at 400 and 101 MHz, respectively, using CDCl3 as the solvent. The HRMS spectrum was recorded using electrospray ionization on a Bruker microTOF apparatus (Bruker Analytische Messtechnik GmbH, Rheinstetten, Germany) in negative mode. All the spectra of the products can be found in the Supplementary Materials.
3.2. Synthesis of 2-Hydroxy-3-octyloxybenzaldehyde
A suspension of NaH (60% dispersion in mineral oil; 0.3 g, 12.5 mmol) in dry DMSO (12.5 mL) was stirred in a cold water bath for 15 min under argon. To the suspension, a solution of 2,3-dihydroxybenzaldehyde (0.69 g, 5 mmol) in dry DMSO (2.5 mL) was added dropwise and the mixture was stirred for another 1 h. Then, 1-bromooctane (0.97 g, 5 mmol) was added in one portion; the color of the solution changed from brown to dark green. The reaction was stirred overnight at room temperature, poured into cold water (60 mL), acidified with 1 M HCl to pH 3 and extracted with petroleum ether. The organic phase was extracted with 1% sodium hydroxide; the aqueous phase was acidified with 1 M HCl to pH 3, then extracted again with petroleum ether, dried over Na2SO4 and evaporated in vacuo.
The resulting residue (0.7 g) was dissolved in EtOH (5 mL); then, a solution of Ni(OAc)2·4H2O (200 mg, 0.81 mmol) in warm EtOH (~2 mL) was added. The mixture was stirred for 30 min at 80 °C. The resulting yellow–green precipitate was filtered, dried and treated with 20% aqueous H2SO4 with the addition of EtOH (2 mL). The resulting mixture was extracted with PE. The organic layer was washed with 1 M NaHCO3 (10 mL), dried over anhydrous Na2SO4, evaporated, dried in vacuo and crystallized in a fridge. The yield was 0.136 g (10.9%).
1H-NMR (400 MHz, CDCl3) δ, ppm: 10.99 (s, 1H), 9.92 (s, 1H), 7.17 (dd, J = 7.9, 1.5 Hz, 1H), 7.11 (dd, J = 8.0, 1.5 Hz, 1H), 6.94 (t, J = 7.9 Hz, 1H), 4.05 (t, J = 6.7 Hz, 2H), 1.85 (t, J = 14.8 Hz, 2H), 1.6–1.2 (m, 10H), 0.89 (t, 3H). 13C-NMR (101 MHz, CDCl3) δ, ppm: 196.6, 152.1, 147.9, 124.7, 121.1, 119.7, 119.6, 69.7, 31.9, 29.5, 29.3, 29.3, 26.1, 22.8, 14.2. FTIR (KBr) ῦ, cm−1: 2800–3000 (C–H, O–H), 1659 (C = O). HRMS (ESI) m/z [M − H]− calcd. for C15H21O3− 249.1496, found 249.1495.
Supplementary Materials
The following are available online: 1H-NMR and 13C-NMR spectral analysis, HRMS and FTIR data for 2-hydroxy-3-octylbenzaldehyde and 1H-NMR spectrum of the crude reaction mixture. Figure S1: 1H-NMR spectrum of 1, CDCl3, 400 MHz. Figure S2: 13C-NMR spectrum of 1, CDCl3, 400 MHz. Figure S3: ESI-HRMS spectrum of 1. Figure S4: FTIR spectrum of 1, KBr. Figure S5: 1H NMR spectrum of reaction mixture with C2H4Br2 additive, CDCl3, 400 MHz.
Author Contributions
Conceptualization: D.A.L. and O.V.L.; Synthesis: J.V.N., A.Y.K. and A.A.V.; Writing—original draft preparation J.V.N.; Writing—review and editing: D.A.L.; Visualization: D.A.L.; Funding acquisition: O.V.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Russian Scientific Foundation, grant nr. 19-19-00175.
Data Availability Statement
Data available on request.
Acknowledgments
We thank the Research Center for Magnetic Resonance, the Center for Chemical Analysis and Materials Research of Saint Petersburg State University Research Park for the measurements provided.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Vereshchagin, A.A.; Lukyanov, D.A.; Kulikov, I.R.; Panjwani, N.A.; Alekseeva, E.A.; Behrends, J.; Levin, O.V. The Fast and the Capacious: A [Ni(Salen)]-TEMPO Redox-Conducting Polymer for Organic Batteries. Batter. Supercaps 2021, 4, 336–346. [Google Scholar] [CrossRef]
- Chepurnaya, I.A.; Karushev, M.P.; Alekseeva, E.V.; Lukyanov, D.A.; Levin, O.V. Redox-conducting polymers based on metal- salen complexes for energy storage applications. Pure Appl. Chem. 2020, 92, 1239–1258. [Google Scholar] [CrossRef]
- Alekseeva, E.V.; Chepurnaya, I.A.; Malev, V.V.; Timonov, A.M.; Levin, O.V. Polymeric nickel complexes with salen-type ligands for modification of supercapacitor electrodes: Impedance studies of charge transfer and storage properties. Electrochim. Acta 2017, 225, 378–391. [Google Scholar] [CrossRef]
- Eliseeva, S.N.; Alekseeva, E.V.; Vereshchagin, A.A.; Volkov, A.I.; Vlasov, P.S.; Konev, A.S.; Levin, O.V. Nickel-Salen Type Polymers as Cathode Materials for Rechargeable Lithium Batteries. Macromol. Chem. Phys. 2017, 218, 1700361. [Google Scholar] [CrossRef]
- Chen, J.; Wagner, P.; Tong, L.; Wallace, G.G.; Officer, D.L.; Swiegers, G.F. A Porphyrin-Doped Polymer Catalyzes Selective, Light-Assisted Water Oxidation in Seawater. Angew. Chem. Int. Ed. 2012, 51, 1907–1910. [Google Scholar] [CrossRef] [PubMed]
- De Castro, B.; Ferreira, R.; Freire, C.; García, H.; Palomares, E.J.; Sabater, M.J. Photochemistry of nickel salen based complexes and relevance to catalysis. New J. Chem. 2002, 26, 405–410. [Google Scholar] [CrossRef]
- Wesley Jeevadason, A.; Kalidasa Murugavel, K.; Neelakantan, M.A. Review on Schiff bases and their metal complexes as organic photovoltaic materials. Renew. Sustain. Energy Rev. 2014, 36, 220–227. [Google Scholar] [CrossRef]
- Konev, A.S.; Kayumov, M.Y.; Karushev, M.P.; Novoselova, Y.V.; Lukyanov, D.A.; Alekseeva, E.V.; Levin, O.V. Polymeric Metal Salen-Type Complexes as Catalysts for Photoelectrocatalytic Hydrogen Peroxide Production. ChemElectroChem 2018, 5, 3138–3142. [Google Scholar] [CrossRef]
- Novozhilova, M.V.; Smirnova, E.A.; Karushev, M.P.; Timonov, A.M.; Malev, V.V.; Levin, O.V. Synthesis and study of catalysts of electrochemical oxygen reduction reaction based on polymer complexes of nickel and cobalt with Schiff bases. Russ. J. Electrochem. 2016, 52, 1183–1190. [Google Scholar] [CrossRef]
- Sukwattanasinitt, M.; Nantalaksakul, A.; Potisatityuenyong, A.; Tuntulani, T.; Chailapakul, O.; Praphairakait, N. An Electrochemical Sensor from a Soluble Polymeric Ni−salen Complex. Chem. Mater. 2003, 15, 4337–4339. [Google Scholar] [CrossRef]
- Martin, C.S.; Dadamos, T.R.L.; Teixeira, M.F.S. Development of an electrochemical sensor for determination of dissolved oxygen by nickel–salen polymeric film modified electrode. Sens. Actuators B Chem. 2012, 175, 111–117. [Google Scholar] [CrossRef]
- Martins, T.S.; Bott-Neto, J.L.; Raymundo-Pereira, P.A.; Ticianelli, E.A.; Machado, S.A.S. An electrochemical furosemide sensor based on pencil graphite surface modified with polymer film Ni-salen and Ni(OH)2/C nanoparticles. Sens. Actuators B Chem. 2018, 276, 378–387. [Google Scholar] [CrossRef]
- Dahm, C.E.; Peters, D.G. Catalytic reduction of α, ω-dihaloalkanes with nickel(I) salen as a homogeneous-phase and polymer-bound mediator. J. Electroanal. Chem. 1996, 406, 119–129. [Google Scholar] [CrossRef]
- Vilas-Boas, M.; Santos, I.C.; Henderson, M.J.; Freire, C.; Hillman, A.R.; Vieil, E. Electrochemical Behavior of a New Precursor for the Design of Poly[Ni(salen)]-Based Modified Electrodes. Langmuir 2003, 19, 7460–7468. [Google Scholar] [CrossRef] [Green Version]
- Keller, F.; Rippert, A.J. New, Axially Chiral, Bimetallic Catalysts for Asymmetric Alkylation of Aldehydes with Diethylzinc. Helv. Chim. Acta 1999, 82, 125–137. [Google Scholar] [CrossRef]
- Malev, V.V.; Levin, O.V.; Timonov, A.M. Quasi-equilibrium voltammetric curves resulting from the existence of two immobile charge carriers within electroactive polymer films. Electrochim. Acta 2013, 108, 313–320. [Google Scholar] [CrossRef]
- Han, S.; Zhang, F.-F.; Qian, H.-Y.; Chen, L.-L.; Pu, J.-B.; Xie, X.; Chen, J.-Z. Design, syntheses, structure–activity relationships and docking studies of coumarin derivatives as novel selective ligands for the CB2 receptor. Eur. J. Med. Chem. 2015, 93, 16–32. [Google Scholar] [CrossRef] [PubMed]
- 3-Substituted Coumarin Derivative and Use Thereof; Shanghai Inst Materia Medica: Shanghai, China; University Zhejiang: Hangzhou, China, 2013; pp. 1–33.
- Liu, D.; Shen, J.; Xu, K.; Zhang, W. Salviandic Acid A Intermediate and Preparation Method Thereof. CN108358761B, 13 March 2018. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).