1. Introduction
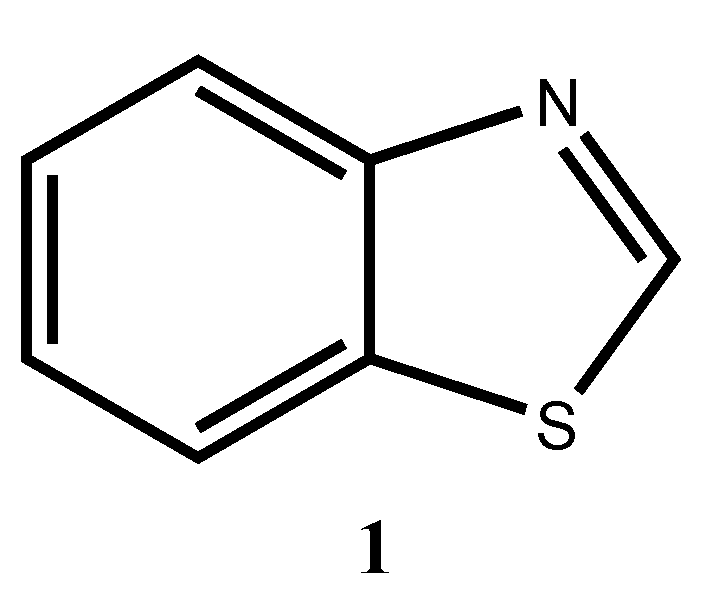
Benzothiazole
1 (
Figure 1) is a heterocyclic organic compound that possesses a wide range of properties and applications. It possesses optical properties, co-ordination properties, electron acceptor properties, etc. [
1] Benzothiazole possesses a vast number of applications as well, including cancer treatment [
2], antibacterial [
3], anticonvulsant [
4], antidiabetic [
3], antifungal [
3], etc.
2-Aminobenzothiazoles are highly reactive molecules and extensively employed as reactants or reaction intermediates for the synthesis of a variety of fused heterocyclic compounds [
5]. The synthesis of C2-substituted benzothiazoles has received much attention. A number of drugs containing the benzothiazole core are commercially used to treat different pathologies. Riluzole
2 or 6-(trifluoromethoxy)benzo[
d]thiazol-2-amine (
Figure 2) owns a neuroprotective action useful in the treatment of amyotrophic lateral sclerosis [
6].
Frentizole
3 or 1-(6-methoxybenzo[
d]thiazol-2-yl)-3-phenylurea (
Figure 2) is another example of a benzothiazole derivative possessing antiviral and immunosuppressant properties [
7].
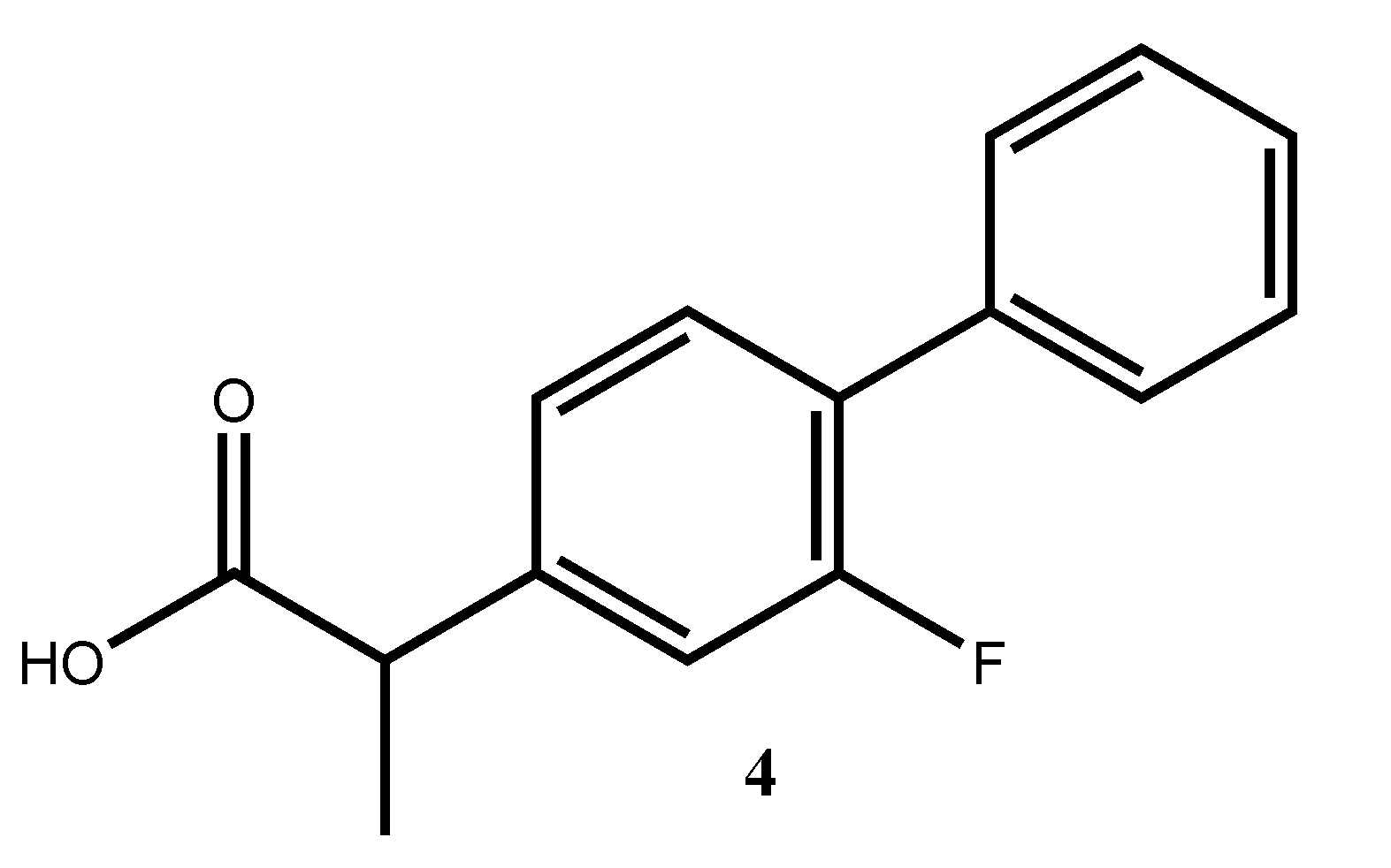
Flurbiprofen
4 (
Figure 3) is a propionic acid derivative and is a non-steroidal anti-inflammatory drug (NSAID). It is known to have anti-inflammatory, analgesic, and anti-pyretic activities, and it is mainly used to treat osteoarthritis and similar diseases [
8].
The synthesis of amides is extremely important for the pharmaceutical industry, where it is estimated that amide preparation is the most common chemical reaction employed [
9]. Approximately a quarter of all marketed drugs and two-thirds of all drug candidates contain a least one amide functional group [
10]. A consideration of how amides interact with biological targets is also a key aspect of drug discovery [
11].
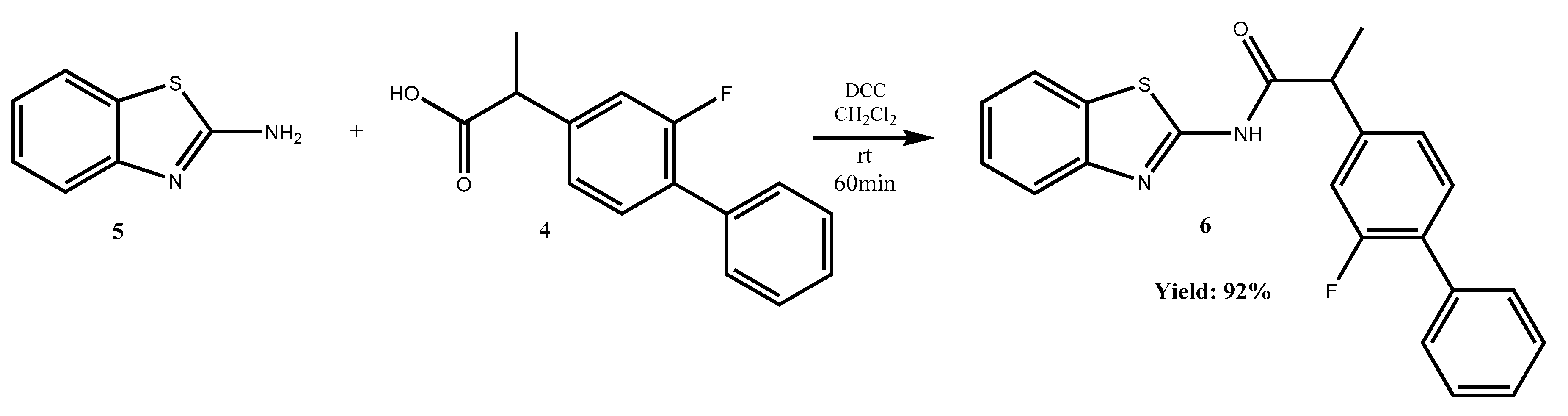
Due to the importance of the amides in pharmaceutical synthesis, a coupling between flurbiprofen and benzo[d]thiazol-2-amine via amide bond formation was achieved in order to obtain N-(benzo[d]thiazol-2-yl)-2-(2-fluoro-[1,1′-biphenyl]-4-yl)propanamide 6.
2. Results
Herein, we report the successfully synthesized
N-(benzo[
d]thiazol-2-yl)-2-(2-fluoro-[1,1′-biphenyl]-4-yl)propanamide
6, as shown in
Scheme 1.
An easy and handy synthetic procedure for amide synthesis is through N,N′-dicyclohexylcarbodiimide (DCC) mediated coupling between amines and carboxylic acids. DCC is a dehydrating agent commonly used for the preparation of esters, anhydrides, and amides. The mechanism of DCC is to activate the carboxylic acid (flurbiprofen) to the nucleophilic amine. This reaction generally works in high yield.
The resultant compound is characterized by its melting point, 1H-NMR and 13C-NMR, UV, IR, and HRMS spectra. The performed NMR analysis fully proves the structure of the obtained molecule. The observed signals of the 1H-NMR spectrum comply with all protons from the molecule. The proton from the NH group is shifted at 12.65 ppm, and the signals for CH (quartet) and CH3 (doublet) from the flurbiprofen part can be seen at 4.12 and 1.52 ppm, respectively. Looking at the 13C-NMR spectrum, a spin–spin interaction with the 19F atom was observed. In this regard, several doublets were present, which are described below in the experimental section. Analyzing the IR spectrum also gives us enough evidence for proving the structure of the analyzed molecule. The transmission of UV light through the amide 6 dissolved in methanol shows the presence of peaks at 255 and 307 nm. The molar absorbency ε at the respective wavelength is also calculated. The HRMS analysis also unambiguously proves the authenticity of the structure.
The used method allows fast and easy synthesis of N-(benzo[d]thiazol-2-yl)-2-(2-fluoro-[1,1′-biphenyl]-4-yl)propanamide 6 in high yield. Successfully obtained amide 6 is synthesized for the first time, and it is interesting because of its potential biological activity. The molecule contains on its structure proven pharmacophores, which are part of used in the medicinal practice medicines.
3. Materials and Methods
All reagents and chemicals were purchased from commercial sources (Sigma-Aldrich S.A. and Riedel-de Haën, Sofia, Bulgaria and used as received. Melting points were determined on a Boetius hot stage apparatus and are uncorrected. The NMR spectral data were recorded on a Bruker Avance II+600 spectrometer (BAS-IOCCP—Sofia, Bruker, Billerica, MA, USA). 1H-NMR and 13C-NMR spectra for compound 6 were taken in DMSO-d6 at 600 MHz and at 150.9 MHz, respectively. Chemical shifts are given in relative ppm and were referenced to tetramethylsilane (TMS) (δ = 0.00 ppm) as an internal standard; the coupling constants are indicated in Hz. The NMR spectra were recorded at room temperature (ac. 295 K). Mass analyses were carried out on a Q Exactive Plus mass spectrometer (Thermo Fisher Scientific, Waltham, MA, USA). TLC was carried out on precoated 0.2 mm Fluka silica gel 60 plates (Merck KGaA, Darmstadt, Germany).
Synthesis of N-(Benzo[d]thiazol-2-yl)-2-(2-fluoro-[1,1′-biphenyl]-4-yl)propanamide 6
N,N′-dicyclohexylcarbodiimide (1 mmol, 0.206 g) was added to a solution of flurbiprofen (1 mmol, 0.244 g) in CH2Cl2. The reaction mixture was stirred at room temperature for 10 min. After the addition of benzo[d]thiazol-2-amine (1 mmol, 0.150 g), the reaction mixture was stirred for 50 min and the formation of white crystalline dicyclohexylurea was observed and then separated by filtration over a sintered glass filter. The filtrate was washed with a diluted hydrochloric acid, a saturated solution of Na2CO3, and brine. The combined organic layers were dried over anhydrous Na2SO4, and the solvent was removed under reduced pressure. The compound was purified by filtration through short column chromatography.
N-(benzo[d]thiazol-2-yl)-2-(2-fluoro-[1,1′-biphenyl]-4-yl)propanamide 6: white solid (m.p. 119–121 °C), yield 92% (0.346 g), 1H-NMR (600 MHz, DMSO-d6) δ 12.65 (s, 1H), 7.99–7.95 (m, 1H), 7.74–7.72 (m, 1H), 7.54–7.49 (m, 3H), 7.48–7.44 (m, 2H), 7.42 (ddd, J = 9.8, 5.4, 4.3 Hz, 1H), 7.40–7.37 (m, 1H), 7.36–7.28 (m, 3H), 4.12 (q, J = 7.0 Hz, 1H), 1.52 (d, J = 7.0 Hz, 3H). 13C-NMR (151 MHz, DMSO-d6) δ 173.16 (C=O), 159.35 (d, 1JC-F = 246.4 Hz), 158.35 (S(N=)C-NH), 148.93 (C, Ar), 142.80 (C, Ar), 135.25 (C, Ar), 131.88 (C, Ar), 131.38 (d, 3JC-F = 3.6 Hz), 131.36 (C, Ar), 129.20 (C, Ar), 129.08 (C, Ar), 128.32 (C, Ar), 126.63 (C, Ar), 124.45 (C, Ar), 124.10 (C, Ar), 122.21 (C, Ar), 121.01 (C, Ar), 115.68 (d, 2JC-F = 23.6 Hz), 44.93 (CH), 18.32 (CH3). UV λmax, MeOH: 255 (ε = 28,300), 307 (ε = 14,400) nm. HRMS Electrospray ionization (ESI) m/z calcd. for C22H18FN2OS+ = 377.1124, found 377.1128 (mass error Δm = 1.06 ppm). IR(KBr) νmax, cm−1: 3115, 3033 ν(Csp2-H), 1701 ν(C=O, secondary amide), 1622 δ(N-H), 1600 ν(C=C, Ph), 1539, δ(N-H) + ν(C-N), 1483 ν(C=C, Ph), 1456 ν(C=C, Ph), δas(CH3), δ(N-CH2), 1265, 1011 ν(Ar-F), 768, 757, 730 γ(Csp2-H), 698, 673 ν(C-S-C).