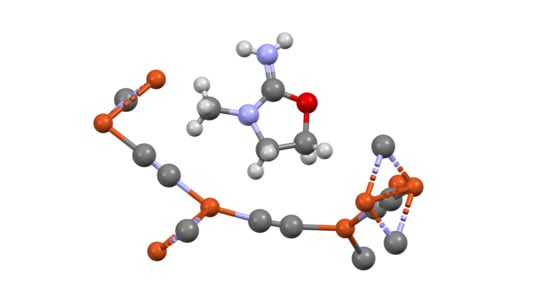
Poly[3-methyl-1,3-oxazolidin-2-iminium[µ3-cyanido-tri-µ2-cyanido-κ9C:N-tricuprate(I)]]
Abstract
:1. Introduction
2. Results
2.1. Synthesis
2.2. X-Ray Structure
2.3. Spectroscopy and Analyses
3. Experimental Section
4. Discussion
5. Materials and Methods
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Koenigsmann, C.; Rachid, L.N.; Sheedy, C.M.; Corfield, P.W.R. Synthesis, decomposition studies and crystal structure of a three-dimensional CuCN network structure with protonated N-methylethanolamine as the guest cation. Acta Crystallogr. Sect. C Struct. Chem. 2020, 76, 405–411. [Google Scholar] [CrossRef] [PubMed]
- Wiley, R.H. The chemistry of the oxazoles. Chem. Rev. 1945, 37, 389–437. [Google Scholar] [CrossRef] [PubMed]
- Lakhan, R.; Ternai, B. Advances in oxazole chemistry. Adv. Heterocycl. Chem. 1974, 17, 99–211. [Google Scholar]
- Turchi, I.J.; Dewar, M.J.S. The Chemistry of Oxazoles. Chem. Rev. 1975, 75, 401–442. [Google Scholar] [CrossRef]
- Turchi, I.J. Oxazole chemistry: A review of recent advances. Ind. Eng. Chem. Prod. Res. Dev. 1981, 20, 32–76. [Google Scholar] [CrossRef]
- Kakkar, S.; Narasimhan, B. A comprehensive review on biological activities of oxazole derivatives. BMC Chem. 2019, 13, 16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Groom, C.R.; Bruno, I.J.; Lightfoot, M.P.; Ward, S.C. The Cambridge Structural Database. Acta Cryst. B 2016, 72, 317–325. [Google Scholar] [CrossRef] [PubMed]
- Rynearson, K.D.; Dutta, S.; Tran, K.; Dibrov, S.M.; Hermann, T. Synthesis of Oxazole Analogs of Streptolidine Lactam. Eur. J. Org. Chem. 2013, 2013, 7337–7342. [Google Scholar] [CrossRef]
- Cruz, A.; Padilla-Martínez, I.I.; García-Báez, E.V.; Contreras, R. Reactivity of Chlorodeoxypseudoephedrines with Oxo-, Thio-, and Selenocyanates. Tetrahedron Asymmetry 2007, 18, 123–130. [Google Scholar] [CrossRef]
- Misaiszek, C.; Jarry, C.; Ouhabi, J.; Carpy, Y. Synthesis and Structural Study of 3-(2-propanone) 5-phenoxymethyl 2-iminooxazolidine: C13H16N2O3HCl. Comptes Rendue L’Academie Sci. Ser. II 1988, 307, 1189–1193. [Google Scholar]
- Satyachand Harisomayajula, N.V.; Makovetskyi, S.; Tsai, Y.-C. Cuprophilic Interactions in and between Molecular Entities. Chem. Eur. J. 2019, 25, 8936–8954. [Google Scholar] [CrossRef] [PubMed]
- Stocker, F.B.; Staeva, T.P.; Rienstra, C.M.; Britton, D. Crystal Structures of a Series of Complexes Produced by Reactions of Copper(I) Cyanide with Diamines. Inorg. Chem. 1999, 38, 984–991. [Google Scholar] [CrossRef]
- Corfield, P.W.R.; Stavola, T.J. Poly[diethylammonium [tetra-µ2-cyanido-κ8C:N-tricuprate(I)]], a two-dimensional network solid. IUCrData 2020, 5, x200968. [Google Scholar] [CrossRef]
- Corfield, P.W.R.; Dayrit, J.R. Poly[1,3-Dimethyltetrahydropyrimidin-2(1H)-iminium [tri-µ2-cyanido-κ6C:N-dicuprate(I)]]. Molbank 2020, 4, M1170. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rachid, L.N.; Corfield, P.W.R. Poly[3-methyl-1,3-oxazolidin-2-iminium[µ3-cyanido-tri-µ2-cyanido-κ9C:N-tricuprate(I)]]. Molbank 2021, 2021, M1259. https://doi.org/10.3390/M1259
Rachid LN, Corfield PWR. Poly[3-methyl-1,3-oxazolidin-2-iminium[µ3-cyanido-tri-µ2-cyanido-κ9C:N-tricuprate(I)]]. Molbank. 2021; 2021(3):M1259. https://doi.org/10.3390/M1259
Chicago/Turabian StyleRachid, Leena N., and Peter W. R. Corfield. 2021. "Poly[3-methyl-1,3-oxazolidin-2-iminium[µ3-cyanido-tri-µ2-cyanido-κ9C:N-tricuprate(I)]]" Molbank 2021, no. 3: M1259. https://doi.org/10.3390/M1259
APA StyleRachid, L. N., & Corfield, P. W. R. (2021). Poly[3-methyl-1,3-oxazolidin-2-iminium[µ3-cyanido-tri-µ2-cyanido-κ9C:N-tricuprate(I)]]. Molbank, 2021(3), M1259. https://doi.org/10.3390/M1259