Abstract
A new compound tetramethyl 1,1′-(2-[{4,5-bis(methoxycarbonyl)-1H-1,2,3-triazol-1-yl}methyl]-2-[(4-methylphenyl)sulfonamido]propane-1,3-diyl)bis(1H-1,2,3-triazole-4,5-dicarboxylate) (3) was prepared in two steps starting from 2-((4-methylphenyl)sulfonamido)-2-((tosyloxy)methyl)propane-1,3-diylbis(4-methylbenzenesulfonate) (1), with an overall yield of 74%. The key step being the copper-free Huisgen cycloaddition between N-(1,3-diazido-2-(azidomethyl)propan-2-yl)-4-methylbenzenesulfonamide (2) and commercially available dimethyl acetylenedicarboxylate. The chemical structure of compound 3 was determined by IR, 1D and 2D NMR experiments, and elemental analysis.
1. Introduction
Although antibiotics remain an effective means against infectious diseases, microbial resistance is a major scourge on global health [1]. This has led to a rush of researchers towards the synthesis of new heterocyclic compounds, justified mainly by their broad spectrum of application. Thus, in recent decades, the 1,2,3-triazoles have become increasingly targeted, due to their interesting antibacterial [2], antioxidant, anti-inflammatory [3], and antifungal properties [4]. They were also used in anti-corrosive treatments [5]. The copper-free Huisgen cycloaddition reaction remains one of the most widely used methods in organic synthesis. It is considered to be the most direct and efficient route to five-membered heterocycles. It is a reaction between a 1,3-dipole and a dipolarophile. The first dipole, the diazoacetic ester, was discovered in 1883 by Curtius [6]. Five years later, Buchner [7] studied the reaction of this dipole with several alkenes, α,β-unsaturated esters and described the first 1,3-dipolar cycloadditions. Since then, many dipoles have been identified and have found general application in synthesis [8,9], notably through the work of Huisgen [10] whose 1,3-dipolar cycloaddition reaction is also carried out between an azide and a terminal alkyne. This cycloaddition reaction has been widely used in our laboratory [11,12,13,14,15,16,17,18,19,20] for the synthesis of new triazole compounds, precursors of heterocyclic amino acids. We cite as examples the diethyl 1-((4-methyl-2-phenyl-4,5-dihydrooxazol-4-yl)methyl)-1H-1,2,3-triazole-4,5-dicarboxylate [17], and the N,N-dibenzyl-1-(1-[(4-methyl-2-phenyl-4,5-dihydrooxazol-4-yl)methyl)]-1H-1,2,3-triazol-4-yl)methanamine [15]. In the same way, we report in this paper the synthesis of a new tritriazolic compound, namely tetramethyl 1,1′-(2-[{4,5-bis(methoxycarbonyl)-1H-1,2,3-triazol-1-yl}methyl]-2-[(4-methylphenyl)sulfonamido]propane-1,3-diyl)bis(1H-1,2,3-triazole-4,5-dicarboxylate) (3). The latter was obtained in two steps from the compound synthesised in the laboratory, the 2-((4-methylphenyl)sulfonamido)-2-((tosyloxy)methyl)propane-1,3-diylbis(4-methylbenzenesulfonate) (1). The approach consisted first to prepare the triazide dipole (2) by nucleophilic substitution of O-tosyl groups of the compound (1) by sodium azide. We then performed a dipolar cycloaddition reaction between the N-(1,3-diazido-2-(azidomethyl)propan-2-yl)-4-methylbenzenesulfonamide (2), and the dimethyl acetylenedicarboxylate. The cycloadduct (3) obtained with an overall yield of 74%, was fully characterized by 1D and 2D NMR, IR, and elemental analysis (Supplementary Materials).
2. Results and Discussion
The starting compound, 2-((4-methylphenyl)sulfonamido)-2-((tosyloxy)methyl)propane-1,3-diyl bis(4-methylbenzenesulfonate) (1) was synthesized from the commercial product 2-amino-2-(hydroxymethyl)propane-1,3-diol (CAS No. [77-86-1]) by the action of six equivalents of tosyl chloride in pyridine at 0 °C for 5 h. The tetratosylated derivative was obtained as pure in 94% yield after recrystallization in anhydrous methanol.
The three O-tosylated groups are then substituted by the azide function via the action of 3,5 equivalents of sodium azide at reflux in acetonitrile for 24 h, leading to the N-tosyl triazide derivative (2) with a yield of 90% after recrystallization in ethyl acetate.
The triazide dipole (2) was carried to reflux in the minimum of toluene with 4,5 equivalents of dimethyl acetylenedicarboxylate (CAS No. [762-42-5]), led after stirring for 72 h to the desired product tetramethyl 1,1′-(2-[{4,5-bis(methoxycarbonyl)-1H-1,2,3-triazol-1-yl}methyl]-2-[(4-methylphenyl)sulfonamido]propane-1,3-diyl)bis(1H-1,2,3-triazole-4,5-dicarboxylate) (3). The N-tosyl tritriazolic derivative (3) was isolated as pure in 82% yield, as a white solid after chromatography on a silica gel column (methylene chloride/ethyl acetate 9/1) and recrystallization in methylene chloride (Scheme 1).
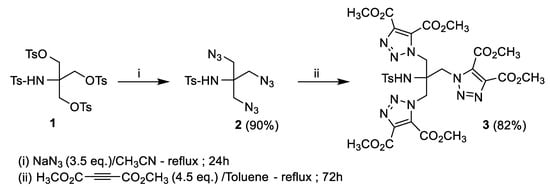
Scheme 1.
Synthetic route for compound (3).
The chemical structure of compound (3) was elucidated by methods of spectroscopic analysis such as 1D and 2D-NMR experiments, infrared spectroscopy, and elemental analysis. The 1H NMR spectrum of the cycloadduct (3) showed the presence of two intense signals at 3.98 and 4.05 ppm corresponding to the 18 methyl protons of the ester groups. Also, the protons of the methylene group of compound (3), which resonate at 5.12 ppm, are more deshielded than those of the triazide derivative (2), which resonate at 3.52 ppm. All of this can be explained by the change in their chemical environment due to the tensions of the triazole rings. Furthermore, in the 13C-NMR spectrum of compound (3), the assignment of the signals appearing at about 159.14 ppm and 159.97 ppm to the quaternary carbons of the carbonyl groups, and those appearing at about 132.48 ppm and 139.57 ppm to the quaternary carbons of the 1,2,3-triazole ring, also confirm that the 1,3-dipolar cycloaddition reaction has taken place. In addition, the appearance of signals at approximately 52.89 ppm and 54.25 ppm in the case of the tris-triazole derivative (3) confirms the presence of methoxy groups of the ester function. The definite assignment of the chemical shifts of protons and carbons are shown in Table 1. The interpretation of the homonuclear and heteronuclear 2D-NMR spectra (Figure 1 and Figure 2) of the cycloadduct (3) showed a perfect correlation, proton-proton and proton-carbon 13.

Table 1.
1H (300 MHz) and 13C (75 MHz) NMR spectral data for compound (3) in CDCl3, including results obtained by homonuclear 2D shift-correlated and heteronuclear 2D shift-correlated HMBC. Chemical shifts (δ in ppm) and coupling constants (J in Hz).
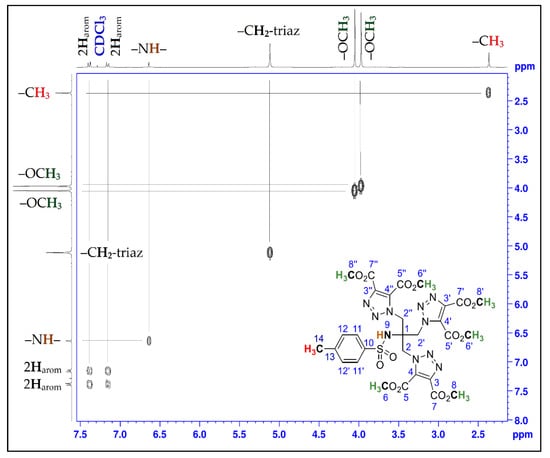
Figure 1.
2D COSY spectrum of compound (3).
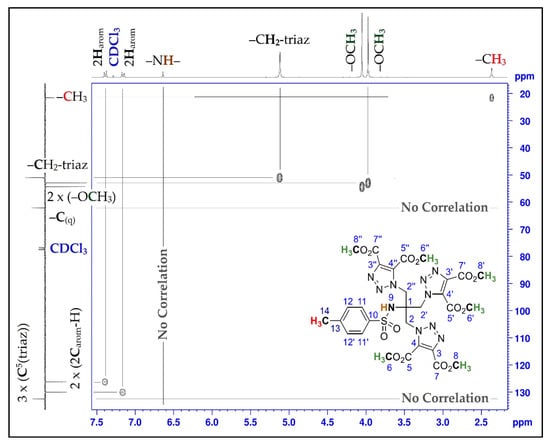
Figure 2.
2D HSQC spectrum of compound (3).
The IR spectrum of compound (2), shows inter alia, a high-intensity band at 2100 cm−1, characteristic for stretching vibrations of the azide group (-N3). Thus, in the spectrum of compound (3), we marked the absence of this last band and the presence of another at 3600 cm−1, characteristic of triazole ring, and two high-intensity bands at 1700–1750 cm−1, characteristics for vibrations of the carbonyl groups (C=O) of ester fragments. We also note the presence of the two other bands at 1350 cm−1 characteristics for stretching vibrations of the (C-O) bonds of ester functions. All of this clearly confirms that the cycloaddition reaction has been carried out.
3. Materials and Methods
All solvents were purified following the standard techniques and commercial reagents were purchased from Sigma-Aldrich (St. Louis, MO, USA). Melting point was determined with an electrothermal melting point apparatus and was uncorrected. NMR spectra (1H and 13C) were recorded on a Bruker AM 300 spectrometer (operating at 300 MHz for 1H, at 75 MHz for 13C) (Bruker Analytische Messtechnik & GmbH, Rheinstetten, Germany). NMR data are listed in ppm and are reported relative to tetramethylsilane (1H, 13C); residual solvent peaks being used as an internal standard. NMR spectroscopic data were recorded in CDCl3 using as internal standards the residual non-deuterated signal (δ = 7.26 ppm) for 1H NMR and the deuterated solvent signal (δ = 77.16 ppm) for 13C NMR spectroscopy. DEPT spectra were used for the assignment of carbon signals. Chemical shifts (δ) are given in ppm and coupling constants (J) are given in Hz. The following abbreviations are used for multiplicities: s = singlet, and d = doublet. All reactions were followed by TLC. TLC analyses were carried out on 0.25 mm thick precoated silica gel plates (Merck Fertigplatten Kieselgel 60F254) and spots were visualized under UV light or by exposure to vaporized iodine. The FT-IR spectrum was recorded in KBr pellet on a Bruker Vertex 70 FTIR spectrometer. Elemental analysis was performed with a Flash 2000 EA 1112, Thermo Fisher Scientific-Elemental Analyzer (CNRST-Rabat, Rabat, Morocco).
3.1. Synthesis of N-(1,3-Diazido-2-(azidomethyl)propan-2-yl)-4-methylbenzenesulfonamide (2)
To 10 mL acetonitrile, 1.3 mmol (958.1 mg) of product (1) and 4.55 mmol (295.7 mg) (3,5 eq.) of sodium azide (CAS No. [26628-22-8]) were added and the mixture is refluxed under stirring for 24 h at 80 °C. At the end of the reaction, the mixture was filtered and then concentrated under vacuum. The residue was washed with water then dried in a vacuum desiccator under P2O5. The obtained product was recrystallized in ethyl acetate.
Yield = 90% (white solid); m.p. = 68–70 °C, Rf = 0.48 (ether/hexane: 2/3). 1H-NMR (CDCl3, δH ppm, 300 MHz): 2.46 (s, 3H, -CH3); 3.52 (s, 6H, 3 × (-CH2-N3)); 5.59 (s, 1H, -NH-); 7.37 (d, 3J = 7.8 Hz, 2Harom); 7.87 (d, 3J = 8.4 Hz, 2Harom). 13C RMN δC (ppm, CDCl3, 75 MHz): 21.6 (1C, -CH3); 52.3 (3C, 3 × (-CH2-N3)); 61.3 (1C, -Cq(NH)); 126.7 (2C, 2 × (Carom-H)); 129.9 (2C, 2 × (Carom-H)); 138.5 (1C, Carom-CH3); 144.2 (1C, Carom-SO2). IR (ν (cm−1)): 3250 (=CH); 2940 (N-H); 2100 (-N3); 1580 (N-H(bending)); 1350 (S=O(stretching)); 780 (=CH).
3.2. Synthesis of Tetramethyl 1,1′-(2-[{4,5-bis(Methoxycarbonyl)-1H-1,2,3-triazol-1-yl}methyl]-2-[(4-methylphenyl)sulfonamido]propane-1,3-diyl)bis(1H-1,2,3-triazole-4,5-dicarboxylate) (3)
In the minimum of toluene (~5 mL), 2 mmol (700.0 mg) of triazide derivative (2) and 9 mmol (1278.0 mg) of dimethyl acetylenedicarboxylate (CAS No. [762-42-5]) were dissolved. The mixture was heated under reflux with stirring for three days. At the end of the reaction, the solvent was removed under reduced pressure and the crude reaction was purified by chromatography on a silica gel column (eluent: methylene chloride/ethyl acetate 9/1). The product thus isolated was recrystallized in methylene chloride.
Yield = 82% (white solid); m.p. = 176–177 °C, Rf = 0.47 (ethyl acetate/ methylene chloride 7/3). 1H-NMR (CDCl3, δH ppm, 300 MHz): 2.37 (s, 3H, -CH3); 3.97 (s, 9H, 3 × (-OCH3)); 4.05 (s, 9H, 3 × (-OCH3)); 5.12 (s, 6H, 3 × (-CH2-triaz)); 6.64 (s, 1H, -NH-); 7.16 (d, 3J = 8.25 Hz, 2Harom); 7.38 (d, 3J = 8.34 Hz, 2Harom). 13C-NMR (CDCl3, δC ppm, 75 MHz): 21.5 (1C, -CH3); 50.9 (3C, 3 × (-CH2-triaz)); 52.9 (3C, 3 × (-OCH3)); 54.3 (3C, 3 × (-OCH3)); 62.1 (1C, -Cq(NH)); 126.1 (2C, 2 × (Carom-H)); 130.0 (2C, 2 × (Carom-H)); 132.5 (3C, 3 × (C5(triaz)); 138.4 (1C, Carom-CH3); 139.6 (3C, 3 × (C4(triaz)); 144.3 (1C, Carom-SO2); 159.1 (3C, 3 × CO); 160.0 (3C, 3 × CO). IR (ν (cm−1)): 3600 (triazole ring); 3250 (=CH); 2900 (N-H); 1700–1750 (C=O two bands); 1580 (N-H(bending)); 1350 (C-O two bands); 1300 (S=O(stretching)); 820 (=CH). Anal. Calcd. for C29H32N10O14S (%): C, 44.85; H, 4.15; N, 18.03; Found (%): C, 44.74; H, 4.18; N, 18.07.
4. Conclusions
The synthesis of the title compound, tetramethyl 1,1′-(2-[{4,5-bis(methoxycarbonyl)-1H-1,2,3-triazol-1-yl}methyl]-2-[(4-methylphenyl)sulfonamido]propane-1,3-diyl)bis(1H-1,2,3-triazole-4,5-dicarboxylate) (3), was carried out with a good yield, via 1,3-dipolar cycloaddition reaction between N-(1,3-diazido-2-(azidomethyl)propan-2-yl)-4-methylbenzenesulfonamide and dimethyl but-2-ynedioate. The characterization of its structure was performed by 1D and 2D NMR spectroscopy, IR, and elemental analysis. The evaluation of the anti-corrosion and biological activities of the synthesized product is under way.
Supplementary Materials
The following data are available online: Figure S1: 1H-NMR spectrum of compound (1), Figure S2: 13C-NMR spectrum of compound (1), Figure S3: 2D COSY spectrum of compound (1), Figure S4: 2D COSY spectrum (aromatic part) of compound (1), Figure S5: 2D HSQC spectrum of compound (1), Figure S6: 2D HSQC spectrum (aromatic part) of compound (1) Figure S7: IR spectrum of compound (3): Figure S8: 1H-NMR spectrum of compound (2), Figure S9: 13C-NMR spectrum of compound (2); Figure S10: 2D COSY spectrum of compound (2), Figure S11: 2D HSQC spectrum of compound (2); Figure S12: IR spectrum of compound (2); Figure S13: 1H-NMR spectrum of compound (3), Figure S14: 13C-NMR spectrum of compound (3), Figure S15: 2D COSY spectrum of compound (3), Figure S16: 2D HSQC spectrum of compound (3), Figure S17: IR spectrum of compound (3).
Author Contributions
S.A.K.F. and S.H. performed the experiments; H.F. and A.A. conceived and designed the experiments; O.K., S.B., Y.A. and A.A. analyzed the data; Y.A. and A.A. wrote the paper. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available in the Supplementary Materials.
Acknowledgments
This work was supported by Sidi Mohammed Ben Abdellah University (USMBA) and National Center for Scientific and Technical Research (CNRST).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Zarei, S.; Komeili, G.; Bahadorikhalili, S.; Yahya-Meymandi, A.; Karami-Zarandi, M.; Larijani, B.; Biglar, M.; Sadat Ebrahimi, S.E.; Mahdavi, M. Design, synthesis and antibacterial activity evaluation of novel 2-(4-((1-aryl-1H-1,2,3-triazol-4-yl)methoxy)phenyl)2-(2-oxoazetidin-1-yl)acetamide derivatives. J. Heterocycl. Chem. 2020, 57, 4254–4261. [Google Scholar] [CrossRef]
- Singh, G.; Singh, J.; Singh, A.; Singh, J.; Kumar, M.; Gupta, K.; Chhibber, S. Synthesis, characterization and antibacterial studies of schiff based 1,2,3-triazole bridged silatranes. J. Organomet. Chem. 2018, 871, 21–27. [Google Scholar] [CrossRef]
- Rani, A.; Singh, G.; Singh, A.; Maqbool, U.; Kaur, G.; Singh, J. CuAAC-ensembled 1,2,3-triazole-linked isosteres as pharmacophores in drug discovery: Review. RSC Adv. 2020, 10, 5610–5635. [Google Scholar] [CrossRef]
- Kaushik, C.P.; Luxmi, R.; Kumar, M.; Singh, D.; Kumar, K.; Pahwa, A. One-pot facile synthesis, crystal structure and antifungal activity of 1,2,3-triazoles bridged with amine-amide functionalities. Synth. Commun. 2019, 49, 118–128. [Google Scholar] [CrossRef]
- González-Olvera, R.; Espinoza-Vázquez, A.; Negrón-Silva, G.E.; Palomar-Pardavé, M.E.; Romero-Romo, M.A.; Santillan, R. Multicomponent click synthesis of new 1,2,3-triazole derivatives of pyrimidine nucleobases: Promising acidic corrosion inhibitors for steel. Molecules 2013, 18, 15064–15079. [Google Scholar] [CrossRef] [PubMed]
- Curtius, T. Ueber die Einwirkung von salpetriger Säure auf salzsauren Glycocolläther. Ber. der Dtsch. Chem. Ges. 1883, 16, 2230–2231. [Google Scholar] [CrossRef]
- Buchner, E. Einwirkung von diazoessigäther auf die aether ungesättigter säuren. Ber. der Dtsch. Chem. Ges. 1888, 21, 2637–2647. [Google Scholar] [CrossRef]
- 1,3‐Dipolar cycloaddition chemistry. Volumes 1 and 2. Edited by Albert Padwa. John Wiley and Sons. New York, 1984. Volume 1: XIII + 817 pages. Volume 2: XIII + 704 pages. J. Heterocycl. Chem. 1986, 23, 1899. [CrossRef]
- Suga, H. Synthetic Applications of 1,3‐Dipolar Cycloaddition Chemistry. Towards Heterocycles and Natural Products (Series: Chem-istry of Heterocyclic Compounds, Vol. 59). Angew. Chem. Int. Ed. 2003, 42, 4569–4570. [Google Scholar] [CrossRef]
- Huisgen, R. 1,3-Dipolar cycloadditions. Past and Future. Angew. Chem. Int. Ed. Engl. 1963, 2, 565–598. [Google Scholar] [CrossRef]
- Atmani, A.; El Hallaoui, A.; El Hajji, S.; Roumestant, M.L.; Viallefont, P. From oxazolines to precursors of amino acids. Synth. Commun. 1991, 21, 2383–2390. [Google Scholar] [CrossRef]
- Labriti, B.; El Hallaoui, A.; Elachqar, A.; Alami, A.; El Hajji, S.; Boukallaba, K.; El Bali, B.; Lachkar, M.; Allouchi, H.; Martinez, J.; et al. Synthesis of 2-phenyl-4-methyl-4-((tetrazol-5-yl)methyl) oxazoline. Mor. J. Heterocycl. Chem. 2006, 5, 58–61. [Google Scholar] [CrossRef]
- Aouine, Y.; Faraj, H.; Alami, A.; El Hallaoui, A.; Elachqar, A.; El Hajji, S.; Kerbal, A.; Labriti, B.; Martinez, J.; Rolland, V. Synthesis of new triheterocyclic compounds, precursors of biheterocyclic aminoacids. Mor. J. Heterocycl. Chem. 2008, 7, 44–49. [Google Scholar] [CrossRef]
- Aouine, Y.; Faraj, H.; Alami, A.; El Hallaoui, A.; El Hajji, S.; Labriti, B.; Kerbal, A. Triheterocyclic compounds, oxazolinic precursors of biheterocyclic amino acids, Part II: Phenothiazine derivatives and structural study of regioisomers through 1H-15N 2D NMR HMBC. Mor. J. Heterocycl. Chem. 2014, 13, 39–47. [Google Scholar] [CrossRef]
- Younas, A.; Abdelilah, E.H.; Anouar, A. N,N-Dibenzyl-1-(1-[(4-methyl-2-phenyl-4,5-dihydrooxazol-4-yl)methyl)]-1H-1,2,3-triazol-4-yl)methanamine. Molbank 2014, 2014, M819. [Google Scholar] [CrossRef]
- Achamlale, S.; Alami, A.; Aouine, Y. Structure assignment of N-protected 2-(1H-1,2,3- triazol-1-yl)-glycine derivatives by chemical and spectroscopic methods. Mor. J. Heterocycl. Chem. 2019, 18, 61–69. [Google Scholar] [CrossRef]
- Boukhssas, S.; Aouine, Y.; Faraj, H.; Alami, A.; El Hallaoui, A.; Bekkari, H. Synthesis, characterization, and antibacterial activity of diethyl 1-((4-methyl-2-phenyl-4,5-dihydrooxazol-4-yl)methyl)-1H-1,2,3-triazole-4,5-dicarboxylate. J. Chem. 2017, 2017, 4238360. [Google Scholar] [CrossRef]
- Achamlale, S.; Elachgar, A.; El Hallaoui, A.; Alami, A.; El Hajji, S.; Roumestant, M.L.; Viallefont, P. Synthesis of biheterocyclic α-amino acids. Amino Acids 1999, 17, 149–163. [Google Scholar] [CrossRef] [PubMed]
- Dioukhane, K.; Moussaid, S.; Achamlale, S.; Alami, A.; Kabbour, M.R.; Aouine, Y.; Faraj, H.; Bouksaim, M.; Gaye, M.L. Comparative study of antibacterial activity of some biheterocyclic “triazolic-tetrazolic” α-amino acid derivatives against bacterial strains. Mor. J. Heterocycl. Chem. 2020, 19, 87–91. [Google Scholar] [CrossRef]
- Hajib, S.; Ksakas, A.; Aouine, Y.; Alami, A.; Faraj, H. Synthesis and structural determination of two new tri-heterocyclic regioisomeric compounds, precursors of bi-triazolic α-amino acids, via a comparative study using 1D NMR Spectroscopy. Eur. J. Adv. Chem. Res. 2020, 1, 1–6. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).