Abstract
4,4′-(Pyrrolo[3,2-b]pyrrole-1,4-diyl)dianiline was synthesized in one step from benzene-1,4-diamine and ethylene glycol with Pd/Al2O3 and ZnO. The title compound was characterized by means of NMR techniques and HRMS mass spectrometry.
1. Introduction
Nitrogen-containing heterocycles, such as pyridine, quinoline, and pyrrole, are found to be present in the vast majority of drugs; therefore, they are considered to be distinctive building blocks in the pharmaceutical industry. An analysis of the database of U.S. FDA approved drugs has pointed out that 59% of small molecule drugs contain a nitrogen-containing heterocycle [1].
In previous works employing ortho or meta benzene-diamine 1 (o, m), compounds 2 and 3 were prepared in excellent yields [2,3] (Scheme 1). Diamine 1 reacted with ethylene glycol, with Pd/C or Pt/Al2O3 as the catalyst. Importantly, ZnO was also required as the co-catalyst. These reactions were performed at 150 or 175 °C during 24–48 h. The formation of compounds 2 and 3 was explained by Borrowing Hydrogen [4,5,6] /Hydrogen Autotransfer [7] (BH/HA)-based mechanisms. Specifically, Acceptorless Dehydrogenative Condensation (ADC) [8,9] is responsible for the formation of those compounds. In this work, we report the results obtained with the para isomer of diamine 1.
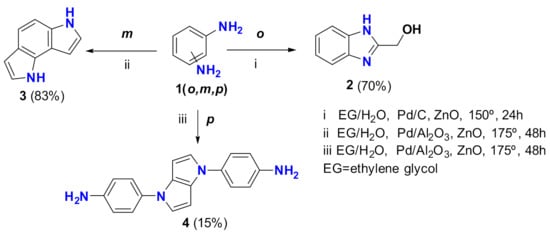
Scheme 1.
Previous results and product obtained in this work.
2. Results
The reaction between benzene-1,4-diamine (1) with ethylene glycol/water in the conditions described by our research group resulted in intractable mixtures. However, when neat ethylene glycol was used as the solvent, we isolated by chromatography an oil that HRMS gives, with a molecular ion at 289.1442 amu corresponding to a C18H16N4 formula. The 1H NMR showed two AB systems, one with two doublets at 6.71 and 7.27 ppm (J = 9 Hz, 8H), and the second at 6.27 and 6.96 ppm (J = 3 Hz, 4H). In the 13C NMR, only seven aromatic signals were observed—four CH and three quaternary carbons. Bidimensional correlations (H,H COSY, HSQC, and HMBC; see Supplementary Materials) allowed the structural elucidation of this compound as 4,4′-(pyrrolo [3,2-b]pyrrole-1,4-diyl)dianiline (4). Pyrrolo[3,2-b]pyrrole-containing compounds have many important applications in different fields. [10] The compound 4 was formed with two units of benzene-1,4-diamine and three units of ethylene glycol. A plausible mechanism based on an ADC process is proposed in Scheme 2.
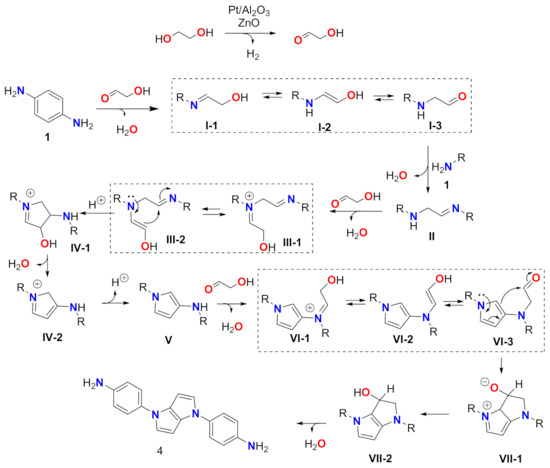
Scheme 2.
Mechanism proposal. Dashed rectangles indicate tautomeric equilibriums.
As can be seen, the dehydrogenation of ethylene glycol provides in situ glyceraldehyde. Condensation with 1 affords imine I-1. Intermediates I-1, I-2, and I-3 are under tautomeric equilibrium. A second imine formation between amino aldehyde I-3 and 1 provides intermediate II. After this, a second molecule of glyceraldehyde reacts to produce III-1. III-1 and III-2 are, again, under tautomeric equilibrium; however, III-2 can undergo intramolecular enamine/imine addition, yielding cyclic intermediate IV-1. Water elimination affords structure IV-2 that, after deprotonation, generates amino pyrrole V. A third molecule of glyceraldehyde produces VI-1 that also presents a tautomeric equilibrium towards VI-2 and VI-3. Intramolecular electrophilic aromatic substitution in VI-3 generates intermediate VII-1, after re-aromatization of VII-2 compound 4 is obtained. A final water elimination yields compound 4. The whole reaction involves two molecules of 1 and three of ethylene glycol. Water and molecular hydrogen are the unique side products. The overall yield was low (15%); however, by thin layer chromatography and NMR, unreacted diamine 1 could be identified.
3. Materials and Methods
Starting materials, if commercially available, were purchased and used as such. ZnO nanoparticles were purchased from Sigma Aldrich (Europe) (<100 nm particle size (DLS), <0 nm average particle size (APS), 20 wt % in H2O). 1H and 13C nuclear magnetic resonance (NMR) spectra were recorded at 300 and 75 MHz in CDCL3. Chemical shifts are reported in δ units, parts per million (ppm), and were measured relative to the signals for residual chloroform. Coupling constants (J) are given in Hertz (Hz). Multiplicities are abbreviated as follows: singlet (s), doublet (d), triplet (t), quartet (q), and multiplet (m). HRMS were recorded using TOF electrospray ionization (ESI-positive). IR spectra were recorded using FT-IR ATR, the solvents used were of spectroscopic or equivalent grade. Water was twice distilled and passed through a Millipore apparatus. All reaction mixtures were filtered through a 0.45 μm PTFE 25 mm syringe filter.
Synthesis of 4,4′-(Pyrrolo[3,2-b]pyrrole-1,4-diyl)dianiline (4)
Benzene-1,4-diamine 1 (2 mmol), 1.7% of Pt/Al2O3 (132.5 mg), 4.5% of ZnO nanoparticles (21.5 μL), and ethylene glycol (4 mL) were mixed manually inside a 50 mL quick-thread glass reaction tube. The tube was sealed with an Easy-On PTFE cap and put into a Carousel 12 Plus reaction station at 175 °C for 48 h. The reaction mixture was cooled to room temperature, and it needed to be opened carefully to depressurize the tube. After that, 25 mL of ethyl acetate was added, and the crude was filtered through a 0.45 μm PTFE filter. The reaction mixture was extracted with distilled water (3 × 10 mL), and the organic layer was dried with Na2SO4, filtered, and concentrated to afford the reaction crude that was checked by 1H-NMR. The crude reaction product was purified by chromatography (Silica gel, Hexane/Ethyl Acetate from 3:1 to 1:3 v:v) as a brown oil identified as the 4,4′-(pyrrolo[3,2-b]pyrrole-1,4-diyl)dianiline (4) (47 mg, yield 15%). 1H-NMR (300 MHz, Cl3CD); δ: 7.26 (d, J = 9 Hz, 4H), 6.95 (d, J = 3 Hz, 2H), 6.70 (d, J = 9 Hz, 4H), 6.27 (d, J = 3 Hz, 2H), 0.7 (sa,4H). 13C-NMR (75 MHz, Cl3CD); δ: 143.4(C), 133.2(C), 128.1(C), 121.3(CH), 121.1(CH), 116.1(CH), 93.6(CH). HRMS: [M + H+] calc. for C18H16N4: 289.1448; found: 289.14425. IR (ATR): 3771, 2922, 2851, 1632, 1516, 1421, 1273, 1177, 825, 689 cm−1. UV-vis (5 × 10−4 M in methanol): λmax 206, 240, and 298 nm.
Supplementary Materials
The following are available online. Figure S1: 1H NMR spectrum of 4, Figure S2: 13C NMR and DEPT 135 spectrum of 4, Figure S3: H, H COSY of 4, Figure S4: HMBC of 4. Figure S5 HSQC of 4. Figure S6 HRMS Mass spectrum of 4. Figure S7 IR (ATR) of 4. Figure S8: UV/Vis Spectra of 4.
Author Contributions
All authors contributed equally to this work. All authors have read and agreed to the published version of the manuscript.
Funding
This work was financially supported by the Generalitat Valenciana (Prometeo/2015/002) and the Universitat de València (Spain) (UV-INV_AE19-1205699).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Vitaku, E.; Smith, D.T.; Njardarson, J.T. Analysis of the structural diversity, substitution patterns, and frequency of nitrogen heterocycles among U.S. FDA approved pharmaceuticals. J. Med. Chem. 2014, 57, 10257–10274. [Google Scholar] [CrossRef] [PubMed]
- Llabres-Campaner, P.J.; Ballesteros-Garrido, R.; Ballesteros, R.; Abarca, B. β-Amino alcohols from anilines and ethylene glycol through heterogeneous Borrowing Hydrogen reaction. Tetrahedron 2017, 73, 5552–5561. [Google Scholar] [CrossRef]
- Llabres-campaner, P.J.; Ballesteros-Garrido, R.; Ballesteros, R.; Abarca, B. Straight Access to Indoles from Anilines and Ethylene Glycol by Heterogeneous Acceptorless Dehydrogenative Condensation. J. Org. Chem. 2018, 83, 521–526. [Google Scholar] [CrossRef] [PubMed]
- Watson, A.J.A.; Williams, J.M.J. The give and take of alcohol activation. Science 2010, 329, 635–636. [Google Scholar] [CrossRef] [PubMed]
- Hamid, M.H.S.A.; Slatford, P.A.; Williams, J.M.J. Borrowing hydrogen in the activation of alcohols. Adv. Synth. Catal. 2007, 349, 1555–1575. [Google Scholar] [CrossRef]
- Yang, Q.; Wang, Q.; Yu, Z. Substitution of alcohols by N-nucleophiles via transition metal-catalyzed dehydrogenation. Chem. Soc. Rev. 2015, 44, 2305–2329. [Google Scholar] [CrossRef] [PubMed]
- Guillena, G.; Ramón, D.J.; Yus, M. Hydrogen autotransfer in the N-alkylation of amines and related compounds using alcohols and amines as electrophiles. Chem. Rev. 2010, 110, 1611–1641. [Google Scholar] [CrossRef] [PubMed]
- Hille, T.; Irrgang, T.; Kempe, R. Synthesis of meta-Functionalized Pyridines by Selective Dehydrogenative Heterocondensation of β-and γ-Amino Alcohols. Angew. Chem. Int. Ed. 2017, 56, 371–374. [Google Scholar] [CrossRef] [PubMed]
- Kallmeier, F.; Dudziec, B.; Irrgang, T.; Kempe, R. Manganese-Catalyzed Sustainable Synthesis of Pyrroles from Alcohols and Amino Alcohols. Angew. Chem. Int. Ed. 2017, 56, 7261–7265. [Google Scholar] [CrossRef] [PubMed]
- Tasior, M.; Vakuliuk, O.; Koga, D.; Koszarna, B.; Górski, K.; Grzybowski, M.; Kielesiński, Ł.; Krzeszewski, M.; Gryko, D.T. The Method for the Large Scale Synthesis of Multifunctional 1,4-Dihydro-pyrrolo[3,2-b]pyrroles. J. Org. Chem. 2020, 85, 13529–13543. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).