Abstract
A new adamantan-2-ol with a 2-bicyclo[4.2.0]octa-1,3,5-trien-3-yl substituent in the position 2 was synthesized via two stage synthesis starting from benzocyclobutene and adamatan-2-one. The structure of the title compound was determined using 1H-and 13C-NMR, HRMS and XRD.
1. Introduction
Adamantanes are used in various fields of science such as material chemistry and medicine. Their inclusion into polymer structure gives materials with excellent properties. For example, adamatan-1-yl acrylate, methacrylate, and 1-vinyl-adamantane-based polymers and polymers containing pendant diamondoid moieties (adamantane and diamantane) are soluble, colorless polymers with an unusual combination of moderately high refractive index (1.48–1.60) and low optical dispersion [1]. These properties make the polymers valuable as optical plastics for lenses, viewfinders, data storage media, light-diffusing elements, etc. [1] It was found that the introduction of adamantyl moieties in poly(1-adamantyl acrylate) increase thermal stability of the polymer in comparison with poly (methyl methacrylate) (PMMA) [2]. A similar result was obtained for 1,3-bis(4-phenyl)adamantane based polysiloxane P1 [3] that has thermal stability (Td5) higher than 520 °C in N2. Introduction of adamantyl moieties in polymer chain in 1,3-bis(4-phenyl)adamantane based polysiloxane P1 also increases glass transition temperature (Tg) to 105–115 °C, more than 125 °C higher than that of poly(tetramethyl-1,4-silphenylenesiloxane) analog [3].
Adamantyl is a non-polar substituent, which should contribute to the improvement of dielectric properties in polymers. This is a very useful characteristic for insulation materials. The development of microelectronics requires high performance interlevel dielectric materials with an extremely low dielectric constant and loss factor.
Adamantane and benzocyclobutene (BCB)-based polymer materials have attracted significant attention because of their low-dielectric constant, low loss factor, and excellent high-temperature performance. An example of such material is AdaDBDVS [4]. AdaDBDVS has Tg = 350 °C, Td5% = 449 °C and dielectric constant (K) = 2.78.
The combination of benzocyclobutene and adamantane in a monomer or polymer seems attractive because a benzocyclobutene ring opens when heated above 200 °C and gives an active isomer (o-xylylene) that provides crosslinking of the polymer. BCB crosslinked polymers have high values of glass transition temperatures (that is relatively low for linear, non-crosslinked adamantane polymers) and high thermal stability [5,6,7].
In this study, we synthesized benzocyclobutyl and adamantyl containing hybride molecule—(2-(bicyclo[4.2.0]octa-1,3,5-trien-3-yl)-adamantan-2-ol) (5), which have free alcohol groups for further modifications. The obtained material can be useful for the developments of new materials with good thermal stability and dielectric properties.
2. Results and Discussion
Synthesis of the title compound is presented in Scheme 1 and includes a three-stage process starting from adamantane (1) and benzocycobutene (3). Adamantan-2-one (2) was obtained from 1 by oxidation by hot sulfuric acid for 25 h [8]. 4-Brombenzocyclobutene (4) was obtained from 3 by bromination in water solution [9,10,11]. A reaction of adamantan-2-one (2) with a Grignard reagent synthesized from 4 gave 5 with 70% yield.
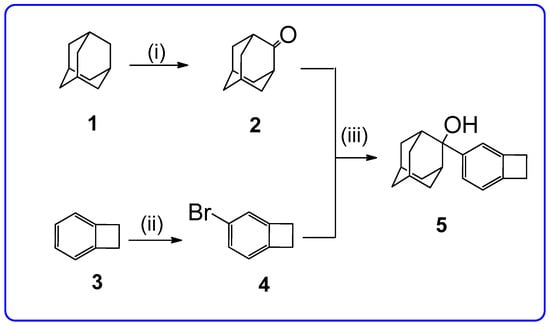
Scheme 1.
Synthesis of 5. (i) H2SO4, heating, 25 h; (ii) Br2, H2O, −10–20 °C, 20 h; (iii) (a) Mg, THF, 25–40 °C, 1 h; (b) 2, 0–5 °C, overnight.
All compounds were characterized by 1H, 13C NMR, and high-resolution mass spectrometry (HRMS). Structure of 5 was also confirmed by the X-ray diffraction method (XRD) (Figure 1A,B). The NMR data of intermediates and title product are presented in the Supplementary Materials.
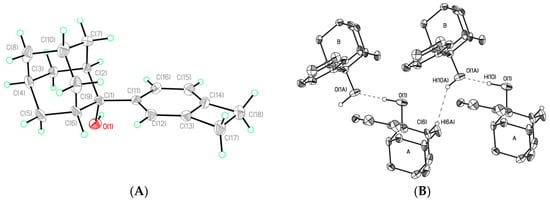
Figure 1.
(A) The general view of one of the independent molecules of 5 in representation of atoms by thermal ellipsoids (p = 50%). Selected bond lengths (A): O(1A)–C(1)1.460(4), C(11)–C(12) 1.405(5), C(11)–C(16) 1.412(5), C(12)–C(13)1.399(5), C(13)–C(14) 1.386(5), C(14)–C(15) 1.387(5), C(14)–C(17) 1.527(5), C(15)–C(16) 1.382(5), C(15)–C(18)1.518(5), C(17)–C(18) 1.579(6); (B) OH…O bonded dimers in the crystal of 5.
The bond lengths and bond angles in 5 have typical values for this class of compounds [12] (Figure 1). According to XRD, 5 crystallizes with two independent molecules (Z’ = 2) in the non-centrosymmetric (chiral) space group P21. Taking into account that two independent molecules in 5 are characterized by the opposite configuration of the C(1) asymmetric center, we can conclude that 5 is a rather rare example of quasi-racemate [13].
The conformations of two independent molecules (A and B) in 5 are almost identical, thus leading to the presence of the non-crystallographic center of symmetry. The main difference between the two enantiomers in 5 is the slight variation of HO-C-C torsion angles. This variation is the consequence of different roles of OH-groups in the supramolecular organization in 5. The OH group of one of the independent molecules (A) participate in the formation of OH…OH hydrogen bond (O(1)-H(1O)…O(1A), H(1O)…O(1A) 2.15 Å, OHO 175, O(1)…O(1A) 2.969(5) Å) with the other molecule (B), while the hydrogen atom of the OH group of the molecule B participates only in the formation of the rather weak and unusual OH…H-C interaction (H…H 2.23 Å, OHH 143°), with the shortest O-H…O intermolecular distance as much as 3.723(5) Å (Figure 1).
3. Materials and Methods
NMR spectra were recorded on a Bruker AM-300 or a Bruker Avance 600 spectrometer (Bruker Corporation, Billerica, MA, USA) in CDCl3. Mass spectra were obtained on a Varian MAT CH-6 instrument (Varian, Inc, Palo Alto, CA, USA) using a direct inlet system; the ionization energy was 70 eV; the acceleration voltage was 1.75 kV. The reaction mixtures were analyzed and the purity of all products was checked by TLC on Merck Silica gel 60 F254 UV-254 plates.
2-(Bicyclo[4.2.0]octa-1,3,5-trien-3-yl)-adamantan-2-ol (5)
4-Bromobenzocyclobutene (1.22 g, 6.7 mmol) was added dropwise to Mg (160 mg, 6.7 mmol) and a crystal of I2 in THF (30 mL) under Ar atmosphere at a temperature of 25–40 °C. Magnesium was completely dissolved after 1 h of stirring. Adamantan-2-one (1.0 g, 6.7 mmol) in THF (7 mL) was added to the resulting Grignard reagent and the mixture was stirred overnight. Water (20 mL) was added to the reaction mixture that was then extracted with ethyl acetate (3 × 20 mL), dried and evaporated. The resulting crude product 5 was purified by flash chromatography to give 5 (1.23 g, 73%) as colorless crystals. 5: m.p 76–77 °C. 1H NMR (500 MHz, CDCl3) δ 7.43 (d, J = 7.7 Hz, 1 h), 7.29 (s, 1 h), 7.09 (d, J = 7.8 Hz, 1 h), 3.20 (s, 4 h), 2.58 (m, 2 h), 2.43 (m, 2 h), 1.95–1.85 (m, 1 h), 1.85–1.65 (m, 9 h). 13C NMR (75 MHz, CDCl3) δ 146.0, 144.8, 144.2, 123.9, 122.7, 119.7, 76.0 (COH), 39.2, 37.7, 35.8, 34.9, 33.1, 29.5, 29.4, 27.5, 27.0. HRMS, found (M+ − H2O): 237.1639, calculated (M+ − H2O): 237.1639.
Crystals of 5 [C18H22O, MW = 254.35] were grown by a slow evaporation of chloroform solution and are monoclinic, space group P21, at 120(2) K a = 6.3740(8), b = 19.523(2), c = 11.3239(14) Å, β = 104.066(3), V = 1366.9(3) Å3, Z(Z’) = 4(2), dcalc = 1.236g cm–3, μ(MoKα) = 0.74 сm−1. Intensities of 17,039 reflections were measured with Bruker APEX-II CCD [λ(MoKα) = 0.71072 Å, 2θ < 58°] and 7273 independent reflections (Rint = 0.0607) were used in the further refinement. The structure was solved by the direct method and refined by the full-matrix least-squares technique against F2 in the anisotropic-isotropic approximation. C-H hydrogen atoms in 5 were placed in calculated positions and were refined in the “riding” model with U(H)iso = 1.2Ueq of their parent atoms. The hydrogen atoms of the OH groups were located from the Fourier density synthesis and refined in an isotropic approximation. The refinement converged to wR2 = 0.1368 and goodness-of-fit (GOF) = 0.998 for all independent reflections (R1 = 0.0605 was calculated against F for 5073 observed reflections with I > 2σ (I)). The maximum and minimum values of difference density were 0.262 and −0.253 e Å−3. All calculations were performed using SHELXTL-2017. Cambridge Crystallographic Data Centre contains the supplementary crystallographic data for this paper No. CCDC 1943491. These data can be obtained free of charge via http://www.ccdc.cam.ac.uk/conts/retrieving.html (or from the CCDC, 12 Union Road, Cambridge CB2 1EZ, UK; Fax: +44 1223 336033; E-mail: deposit@ccdc.cam.ac.uk).
4. Conclusions
A new 2-(bicyclo[4.2.0]octa-1,3,5-trien-3-yl)-adamantan-2-ol with a 2-(bicyclo[4.2.0]octa-1,3,5-trien -3-yl) substituent in the 2-position was obtained via a three stage synthesis starting from benzocyclobutene and adamantane. Target product can be useful for the development of new materials with good thermal stability and dielectric properties.
Supplementary Materials
CIF, MOL files, and NMR data of title compound are available online, Figure S1: 1H NMR of compound 4, 4-Bromobenzocyclobutene; Figure S2: 1H NMR of compound 2, Adamantan-2-one; Figure S3: 1H and 13C NMR of compound 5, 2-(bicyclo[4.2.0]octa-1,3,5-trien-3-yl)-adamantan-2-ol.
Author Contributions
Synthesis, K.S.L. and K.A.C.; NMR data analysis, D.Y.D. and P.S.S.; DFT (density functional theory) calculation and visualization, K.A.L.; Writing—original draft preparation, K.S.L.; Writing—review and editing, K.S.L. and P.S.S.; Supervision and project administration, P.S.S. All authors have read and agreed to the published version of the manuscript.
Funding
The work was financially supported by a grant from the Ministry of Science and Higher Education of the Russian Federation (agreement on granting a subsidy from the Ministry of Science and Higher Education of the Russian Federation dated 14 June 2019, No. 075-15-2019-1273 (inner No. 14.577.21.0273), Unique identifier—RFMEFI57717X0273.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References and Notes
- Robello, D.R. Moderately high refractive index, low optical dispersion polymers with pendant diamondoids. J. Appl. Polym. Sci. 2012, 127, 96–103. [Google Scholar] [CrossRef]
- Wang, K.; Cui, R.; Gu, J.; Yu, Q.; Ma, G.; Nie, J. Photopolymerization of 1-adamantyl acrylate pohotoinitiated by free radical photoinitiators. J. Appl. Polym. Sci. 2012, 123, 26–31. [Google Scholar] [CrossRef]
- Hattori, Y.; Miyajima, T.; Sakai, M.; Nagase, Y.; Nemoto, N. Synthesis and thermal characterization of novel adamantane-based polysiloxane. Polymer 2008, 49, 2825–2831. [Google Scholar] [CrossRef]
- Cheng, Y.; Chen, W.; Li, Z.; Zhu, T.; Zhang, Z.; Jin, Y. Hydrolysis and condensation of a benzocyclobutene-functionalized precursor for the synthesis of high performance low-K polymers. RSC Adv. 2017, 7, 14406–14412. [Google Scholar] [CrossRef]
- Levchenko, K.S.; Chudov, K.A.; Adamov, G.E.; Poroshin, N.O.; Shmelin, P.S.; Grebennikov, E.P.; Parshikov, Y.G. Photocurable and Thermosetting Polymer Materials on the Basis of Benzocyclobutene and Its Derivatives for Electronics. Russ. J. Gen. Chem. 2018, 88, 2793–2812. [Google Scholar] [CrossRef]
- Levchenko, К.S.; Chudov, К.А.; Demin, D.Y.; Аdamov, G.Е.; Poroshin, N.О.; Shmelin, P.S.; Grebennikov, Е.P.; Chvalun, S.N.; Zubov, V.P. Synthesis of photo and thermosetting monomers and polymers based on benzocyclobutene. Russ. Chem. Bull. 2019, 68, 1321–1342. [Google Scholar] [CrossRef]
- Kirchhoff, R.A.; Bruza, K.J. Polymers from benzocyclobutenes. In High Performance Polymers; Hergenrother, P.M., Ed.; Advances in Polymer Science; Springer: Berlin/Heidelberg, Germany, 1994; Volume 117, pp. 1–66. [Google Scholar] [CrossRef]
- Geluk, H.W.; Keizer, V.G. Adamantanone. Org. Synth. 1973, 53, 8. [Google Scholar] [CrossRef]
- Tang, P.C.; LÜ, H.; Zheng, H.; Chen, Y.; Fei, H.; Wang, S.; Wang, L. Bicyclo-Substituted Pyrazolon Azo Derivatives, Preparation Process and Pharmaceutical Use Thereof. E.P. Patent 2,236,500B1, June 2010. [Google Scholar]
- 4-Bromobenzocyclobutene (4). Benzocyclobutene (23.7 g, 22.8 mmol) was dispersed in 240 mL of water at room temperature. After cooling with ice water, (−10–5 °C), bromine (11.7 mL) was added dropwise. After complete addition, ice-water bath was removed and the reaction mixture was warmed to room temperature and stirred overnight. The reaction was monitored by TLC until the starting benzocyclobutene disappeared. The mixture was diluted with 50 mL of n-hexane and sodium sulfite (3.0 g, 23.8 mmol) was added. Upon completion of the addition, the mixture was stirred at room temperature for 30 min and becomes clear. Then, the separated organic layer was dried over anhydrous sodium sulfate, filtered to remove a drying agent, and concentrated under reduced pressure to give 4. After distillation at 110–114 °C (15 mm of Hg), compound 4 (28–30 g, 67–71%) was obtained as a colorless liquid. 1H NMR (300 MHz, CDCl3) δ 7.40 (d, J = 7.8 Hz, 1H), 7.26 (s, J = 7.0 Hz, 1H), 6.99 (d, J = 7.8 Hz, 1H), 3.31–3.10 (m, 4H).
- Roth, M.; Ahles, M.; Gawrisch, C.; Schwalm, T.; Schmechel, R.; Melzer, C.; Seggern, H.; Rehahn, M. Rodlike tetracene derivatives. Chem. Eur. J. 2017, 23, 13445–13454. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.-Q.; Fang, W.; Wei, Y.; Tang, X.-Y.; Shi, M. Gold(i)-catalyzed dehydrogenative cycloisomerization of 1,5-enynes. Chem. Commun. 2016, 52, 10799–10802. [Google Scholar] [CrossRef] [PubMed]
- Lyssenko, K.A.; Lenev, D.A.; Kostyanovsky, R.G. Self-assembly of cage structures. Paper 12: The synthesis and crystal structures of 2,5-diazabicyclo[2.2.2]octane-3,6-dione-1,4dicarboxylic acids and their diesters. Tetrahedron 2002, 58, 8525–8537. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).