Abstract
2-(3,4-Dimethoxyphenyl)-N-(4-methoxyphenyl)-1-propyl-1H-benzo[d]imidazole-5-carboxamide was synthesized by the ‘one-pot’ reductive cyclization of N-(4-methoxyphenyl)-3-nitro-4-(propylamino)benzamide with 3,4-dimethoxybenzaldehyde, using sodium dithionite as a reductive cyclizing agent using DMSO as a solvent. The structure of newly synthesized compound was elucidated based on IR, 1H-NMR, 13C-NMR, and LC-MS data.
1. Introduction
Benzimidazole is important class of heterocyclic compounds having an imidazole ring, which represents one of the most biologically active classes of compounds, possessing a wide spectrum of activities that are well documented in literature [1]. Due to its special spectral features and electron rich environment, benzimidazole containing drugs bind to a variety of therapeutic targets. Substituted benzimidazole derivatives are associated with various types of pharmacokinetic and pharmacodynamics properties. These properties of the benzimidazole derivatives is due to their affinity towards the variety of enzymes and the protein receptors. Benzimidazole derivatives act as proton pump inhibitors by accumulating in the parietal cell and binding directly to the enzyme, which results in inhibition of gastric acid secretion [2]. Benzimidazole derivatives show their antibacterial activity by inhibiting the bacterial nucleic acid and proteins synthesis. Benzimidazole derivatives show their antifungal activity by blocking the polymerization of α- and β-tubulin subunits. The benzimidazole disrupt microtubule function in eukaryotic organisms such as fungi, protozoa, and helminths. The antibacterial and antifungal activity of benzimidazole derivatives is due to its close relationship with structure of purines [3]. Thus, benzimidazole derivatives have found a very strong application in the medicine filed. The synthesis and application of benzimidazole and its derivatives is discussed in several articles [4,5]. Optimization of substituents around the benzimidazole nucleus has resulted in many drugs like Albendazole, Mebendazole, Triclabendazole, Fenbendazole, Oxfendazole, Thiabendazole, Omeprazole, Lansoprazole, and Pantoprazole [6]. Benzimidazole and its derivatives have associated with diverse biological activities such as anti-microbial [7,8,9], anti-inflammatory and analgesic [10], anti-tubercular [11], anti-oxidant [12], anti-fungal [13], anti-convulsant/anti-diabetic [14], and anti-viral [15]. Substituted benzimidazole derivatives have found commercial applications in diverse human therapeutics, especially for treating various types of cancers [16,17,18,19,20].
Carboxamide compounds have diversified applications in medicinal chemistry as an important pharmacophore found in many marketed drug molecules [21]. Carboxamide’s potential as a pharmacophore is due to its capability as an H-bond donor and acceptor [22,23]. It is an active unit in many drug molecules due to its property to bind with protein molecules [24]. Various heterocyclic moieties with amide functionality have displayed various potent biological activities as anti-cancer [25,26], anti-convulsant [27], anti-tubercular [28], anti-oxidant [29] and anti-inflammatory [30] activities. ‘One-pot’ synthesis of benzimidazole has gained much attraction in synthetic organic chemistry as it reduces the number of steps involved and the reaction time, and increases the yield of the product [31]. ‘One-pot’ reductive cyclization was found to be highly efficient using sodium dithionite as reductive cyclizing agent compared to other reagents [32,33,34,35]. Thus, in this paper we describe the efficient synthesis and characterization (by IR, 1H-NMR, 13C-NMR, and LC-MS spectral data) of new benzimidazole coupled carboxamide.
2. Results and Discussion
Synthesis of 2-(3,4-dimethoxyphenyl)-N-(4-methoxyphenyl)-1-propyl-1H-benzo[d]imidazole-5-carboxamide 3 is depicted in Scheme 1. Initially, N-(4-methoxyphenyl)-3-nitro-4-(propylamino)benzamide 1 was obtained by the simple acid amine coupling of p-Anisidine and 3-propylamino-2-nitro-benzoic acid. Further, product 3 was obtained by heating the compound 1 with 3,4-dimethoxybenzaldehyde 2 and sodium dithionite (Na2S2O4) as a reducing agent in dimethyl sulphoxide (DMSO) medium at 90 °C for about 3 h. The obtained product 3 was isolated by means of simple water workup and purified by recrystallization process in a DMF (dimethylformamide) solvent.
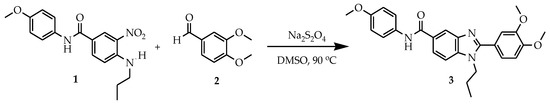
Scheme 1.
Synthesis of 2-(3,4-dimethoxyphenyl)-N-(4-methoxyphenyl)-1-propyl-1H-benzo[d]imidazole-5-carboxamide (3).
The structure of the compound 3 was confirmed by IR, 1H-NMR, 13C-NMR, and LC-MS spectral data. The 1H-NMR spectrum of compound 3 showed a presence of a down-field singlet at δ 10.263 for its -NH proton, indicating the presence of a carboxamide group. The proton ortho to the carbonyl group of amides appeared as a singlet at δ 8.350 ppm and the remaining two protons of the benzimidazole ring appeared as a multiplets in the region of δ 8.028–8.094 ppm. The protons meta and ortho to the methoxy group of the amide phenyl ring appeared as two distinct doublets centered at 7.6865 and 6.9195 ppm with J = 9.2 Hz and 8.4 Hz, respectively. The three protons of the aryl ring at the 2nd position of benzimidazole appeared at 7.229–7.251 and 7.433–7.449 ppm. The presence of triplet at δ 4.410 ppm (J = 7.2 Hz) for its N-CH2 protons, a multiplets at δ 1.735–1.790 ppm for the central -CH2 protons, and another triplet at δ 0.768–0.804 ppm (J = 7.2 Hz) for the -CH3 proton confirms the presence of propyl substituent at the 1st position of benzimidazole. Furthermore, its spectrum showed the presence of three distinct singlet at δ 3.726, 3.838, and 3.865 ppm for their three methoxy protons attached to two phenyl rings. In 13C-NMR spectrum of 3, -C=O carbon of -CONH (carboxamide group) appeared at δ 165.116 ppm, which confirms the presence of the amide carbonyl group. Further, it shows signals for the aromatic carbons at δ 156.039, 151.758, 149.316, 136.640, 132.699, 131.298, 124.050, 123.165, 122.523, 116.753, 114.204, 113.192, 112.414, and 112.278 ppm, which confirms the presence of three aromatic rings in compound 3. In its 13C-NMR spectrum, it shows signals at δ 46.947 ppm, δ 22.810 ppm, and δ 11.251 ppm for the N-CH2, CH2, and CH3 carbon atoms of the propyl chain, respectively. Further, -OCH3 carbons appeared at δ 56.239 and 55.645 ppm, which confirms the presence of three methoxy groups attached to the aromatic ring. Compound 3 was further confirmed by its mass spectra; MS (m/z): 446.1806 [M + H]+ corresponding to its molecular weight.
3. Materials and Methods
3.1. Instruments
All the chemicals used were acquired from Spectrochem (Bangalore, India) and Sigma-Aldrich Company (Bangalore, India). All the solvents and reagents used were dried by standard methods wherever required. The 1H-NMR spectra were recorded on an Agilent-NMR (400 MHz, Agilent, Santa Clara, CA, USA) spectrometer and 13C-NMR spectra was recorded on an Agilent-NMR spectrometer (100 MHz, Santa Clara, CA, USA) with DMSO-d6 as the solvent. Chemical shifts were reported in δ ppm (parts per million) values; multiplicities are indicated by s (singlet), d (doublet), t (triplet), and m (multiplets). Coupling constants (J) were reported in hertz (Hz). The mass spectroscopy of the synthesized compounds was performed on a mass spectrophotometer (MODEL–WATERS, SYNAPT G2 APCI mode, Milford, MA, USA). The progress of the reaction was monitored by thin layer chromatography (TLC) using precoated silica plates 60 F254 aluminium sheets and visualization was done by UV light at 254 nm (UV lamp light 254/365 nm). The melting point was determined by an open capillary method and are uncorrected. The IR spectra were recorded on a Bruker FT-IR spectrophotometer (Bruker, Billerica, MA, USA).
3.2. Synthetic Procedures
The synthetic procedure was optimized based on the trial experiments and the yields were not optimized. The starting material N-(4-methoxyphenyl)-3-nitro-4-(propylamino)benzamide 1 was prepared by coupling of 3-nitro-4-(propylamino)benzoic acid with p-Anisidine using N,N,N’,N’-Tetramethyl-O-(1H-benzotriazol-1-yl)uronium hexafluorophosphate (HBTU) as coupling agent in the presence of N-Methylmorpholine (NMM) base in DMF medium.
Synthesis of 2-(3,4-Dimethoxyphenyl)-N-(4-methoxyphenyl)-1-propyl-1H-benzo[d]imidazole-5-carboxamide (3):
To a solution of N-(4-methoxyphenyl)-3-nitro-4-(propylamino)benzamide 1 (1 eq) in DMSO, 3,4-dimethoxybenzaldehyde (1 eq) 2 and sodium dithionite were added (3 eq). The reaction mass was stirred at 90 °C for 3 h. Completion of the reaction was monitored by thin layer chromatography (TLC) (Hexane:Ethyl acetate; 7:3 v/v). After completion of reaction, the reaction mass was poured into crushed ice. The solid product obtained was filtered, washed with cold water, and dried. Then, compound 3 was recrystallized using dimethylformamide (DMF) solvent.
Molecular formula: C26H27N3O4; Yield: 90%; Melting point: 210–212 °C; IR (KBr, νmax, cm−1): 2999.63–2821.63 (C-H), 1654.42 (C=O); 1H-NMR (400 MHz, δ ppm, DMSO-d6) δ: 0.768–0.804 (t, 3H, J = 7.2 Hz), 1.735–1.790 (m, 2H), 3.726 (s, 3H), 3.838 (s, 3H), 3.865 (s, 3H), 4.392–4.428 (t, 2H,J = 7.2 Hz), 6.909–6.930 (d, 2H, J = 8.4 Hz), 7.229–7.251 (d, 1H, J = 8.8 Hz), 7.433–7.449 (m, 2H), 7.675–7.698 (d, 2H, J = 9.2 Hz), 8.028–8.094 (m, 2H), 8.350 (s, 1H), 10.263 (1H, s); 13C-NMR (100 MHz, DMSO-d6,δ in ppm): δ 165.116, 156.039, 153.743, 151.758, 149.316, 136.640, 132.699, 131.298, 124.050, 123.165, 122.523, 116.753, 114.204, 113.192, 112.414, 112.278 56.239, 55.645, 22.810 and 11.251 ppm; MS (m/z): 446.1806 [M + H]+ (Supplementary Materials).
Supplementary Materials
The following are available online, Figure S1: 1H-NMR of the compound 3, Figure S2: 13C-NMR of the compound 3, Figure S3: LC-MS spectra of the compound 3, Figure S4: IR spectrum of the compound 3.
Author Contributions
The conceptualization, data curation and formal analysis of analytical done by P.B., Ph.D. student under the guidance of S.K.T.H., and V.K. The resources for conducting experiments was arranged by P.B. with help of V.K., and S.H.B.V. Investigation and project administration was done by P.B. with the help of S.H.B.V under the supervision of S.K.T.H. Visualization and writing of initial draft was done by P.B. with help of S.K.T.H., V.K. and S.H.B.V. Writing-review and editing done by P.B. with the help of S.K.T.H. and V.K.
Funding
The authors have not received financial assistance from any funding agencies.
Acknowledgments
The authors are thankful to Jain University for providing opportunity to do research work. Also, we are thankful to University of Mysore for their support in acquiring the analytical data.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Spasov, A.A.; Yozhitsa, I.N.; Bugaeva, L.I.; Anisimova, V.A. Benzimidazole derivatives: Spectrum of pharmacological activity and toxicological properties (a review). Pharm. Chem. J. 1999, 33, 6–17. [Google Scholar] [CrossRef]
- Shin, J.M.; Kim, N. Pharmacokinetics and pharmacodynamics of the proton pump inhibitors. J. Neurogastroenterol. Motil. 2013, 19, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Gurvinder, S.; Maninderjit, K.; Mohan, C. Benzimidazoles: The latest information on biological activities. Int. Res. J. Pharm. 2013, 4, 82–87. [Google Scholar]
- Ansari, N.H.; Söderberg, B.C.G. Synthesis of N-alkoxy-substituted 2H-benzimidazoles. Tetrahedron Lett. 2017, 58, 4717–4720. [Google Scholar] [CrossRef] [PubMed]
- Salahuddin; Mohammad, S.; Mazumder, A. Benzimidazoles: Biologically active compounds (A Review). Arab. J. Chem. 2017, 10, S157–S173. [Google Scholar] [CrossRef]
- Velik, J.; Baliharová, V.; Fink-Gremmels, J.; Bull, S.; Lamka, J.; Skálová, L. Benzimidazole drugs and modulation of biotransformation enzymes. Res. Vet. Sci. 2004, 76, 95–108. [Google Scholar] [CrossRef] [PubMed]
- Desai, N.C.; Shihory, N.R.; Kotadiya, G.M. Facile synthesis of Benzimidazole bearing 2-pyridone derivatives as potential antimicrobial agents. Chin. Chem. Lett. 2014, 25, 305–307. [Google Scholar] [CrossRef]
- Shingalapur, R.V.; Hosamani, K.M.; Keri, R.S. Synthesis and evaluation of in vitro anti-microbial and anti-tubercular activity of 2-styryl benzimidazoles. Eur. J. Med. Chem. 2009, 44, 4244–4248. [Google Scholar] [CrossRef]
- Alasmar, F.A.; Snelling, A.M.; Zain, M.E.; Alafeefy, A.M.; Awaad, A.S.; Karodia, N. Synthesis and evaluation of selected benzimidazole derivatives as potential antimicrobial agents. Molecules 2015, 20, 15206–15223. [Google Scholar] [CrossRef]
- Gaba, M.; Singh, S.; Mohan, C. Benzimidazole: An emerging scaffold for analgesic and anti-inflammatory agents. Eur. J. Med. Chem. 2014, 76, 494–505. [Google Scholar] [CrossRef]
- Kalalbandi, V.K.; Seetharamappa, J.; Katrahalli, U.; Bhat, K.G. Synthesis, crystal studies, anti-tuberculosis and cytotoxic studies of 1-[(2E)-3-phenylprop-2-enoyl]-1H-benzimidazole derivatives. Eur. J. Med. Chem. 2014, 79, 194–202. [Google Scholar] [CrossRef] [PubMed]
- Usta, A.; Yilmaz, F.; Kapucu, G.; Baltas, N.; Mentese, E. Synthesis of Some New Benzimidazole Derivatives with their Antioxidant Activities. Lett. Org. Chem. 2015, 12, 227–232. [Google Scholar] [CrossRef]
- Si, W.; Zhang, T.; Li, Y.; She, D.; Pan, W.; Gao, Z.; Ning, J.; Mei, X. Synthesis and biological activity of novel benzimidazole derivatives as potential antifungal agents. J. Pestic. Sci. 2016, 41, 15–19. [Google Scholar] [CrossRef] [PubMed]
- Shingalapur, R.V.; Hosamani, K.M.; Keri, R.S.; Hugar, M.H. Derivatives of benzimidazole pharmacophore: Synthesis, Anticonvulsant, antidiabetic and DNA cleavage studies. Eur. J. Med. Chem. 2010, 45, 1753–1759. [Google Scholar] [CrossRef] [PubMed]
- Tonelli, M.; Paglietti, G.; Boido, V.; Sparatore, F.; Marongiu, F.; Marongiu, E.; La Colla, P.; Loddo, R. Antiviral activity of benzimidazole derivatives. I. Antiviral activity of 1-substituted-2-[(benzotriazol-1/2-yl)methyl]benzimidazoles. Chem. Biodivers. 2008, 5, 2386–2401. [Google Scholar] [CrossRef] [PubMed]
- Nofal, Z.M.; Soliman, E.A.; Abd El-Karim, S.S.; EI Zahar, M.I.; Srour, A.M.; Sethumadhavan, S.; Maher, T.J. Novel benzimidazole derivatives as expected anticancer agents. Acta Pol. Pharm. 2011, 68, 519–534. [Google Scholar] [PubMed]
- Farmanzadeh, D.; Najafi, M. Benzimidazole derivatives as anticancer drugs: A theoretical investigation. J. Theor. Comput. Chem. 2015, 14, 1550018. [Google Scholar] [CrossRef]
- Bui, H.T.B.; Ha, Q.T.K.; Keun Oh, W.; Vo, D.D.; Chau, Y.N.T.; Thi Kim Tu, C.; Canh Pham, E.; Thao Tran, P.; Thi Tran, L.; Mai, H.V. Microwave assisted synthesis and cytotoxic activity evaluations of new benzimidazole derivatives. Tetrahedron Lett. 2016, 57, 887–891. [Google Scholar] [CrossRef]
- Ibrahim, H.S.; Albakri, M.E.; Mahmoud, W.R.; Allam, H.A.; Reda, A.M.; Abdel-Aziz, H.A. Synthesis and biological evaluation of some novel thiobenzimidazole derivatives as anti-renal cancer agents through inhibition of c-MET kinase. Bioorg. Chem. 2019, 85, 337–348. [Google Scholar] [CrossRef] [PubMed]
- Bhambra, A.S.; Edgar, M.; Elsegood, M.R.J.; Horsburgh, L.; Krystof, V.; Lucas, P.D.; Mojally, M.; Teat, S.J.; Warwick, T.G.; Weaver, G.W.; et al. Novel fluorinated benzimidazole-based scaffolds and their anticancer activity in vitro. J. Fluorine Chem. 2016, 18, 99–109. [Google Scholar] [CrossRef]
- Baumann, M.; Baxendale, I.R. An overview of the synthetic routes to the best selling drugs containing 6-membered heterocycles. Beilstein J. Org. Chem. 2013, 9, 2265–2319. [Google Scholar] [CrossRef] [PubMed]
- Yule, I.A.; Czaplewski, L.G.; Pommier, S.; Davies, D.T.; Narramore, S.K.; Fishwick, C.W.G. Pyridine-3-carboxamide-6-yl-ureas as novel inhibitors of bacterial DNA gyrase: Structure based design, synthesis, SAR and antimicrobial activity. Eur. J. Med. Chem. 2014, 86, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Narramore, S.; Stevenson, C.E.M.; Maxwell, A.; Lawson, D.M.; Fishwick, C.W.G. New insights into the binding mode of pyridine-3-carboxamide inhibitors of E. coli DNA gyrase. Bioorg. Med. Chem. 2019, 27, 3546–3550. [Google Scholar] [CrossRef] [PubMed]
- Nuhrich, A.; Varache-Lembège, M.; Vercauteren, J.; Dokhan, R.; Renard, P.; Devaux, G. Synthesis and binding affinities of a series of 1,2-benzisoxazole-3-carboxamides to dopamine and serotonin receptors. Eur. J. Med. Chem. 1996, 31, 957–964. [Google Scholar] [CrossRef]
- Cai, W.; Liu, A.; Li, Z.; Dong, W.; Liu, X.; Sun, N. Synthesis and anticancer activity of novel Thiazole-5-carboxamide derivatives. Appl. Sci. 2016, 6, 8. [Google Scholar] [CrossRef]
- Kalpana, K.; Kumar, K.R.; Babu, A.V.; Vanjivaka, S.; Vantikommu, J.; Palle, S. Synthesis and biological evaluation of pyrazole amides fused combretastatin derivatives as anticancer agents. Curr. Bioact. Compd. 2018, 14, 357–363. [Google Scholar] [CrossRef]
- Moreau, S.; Coudert, P.; Rubat, C.; Vallee-Goyet, D.; Gardette, D.; Gramain, J.C.; Couquelet, J. Synthesis and anticonvulsant properties of triazolo- and imidazopyridazinyl carboxamides and carboxylic acids. Bioorg. Med. Chem. 1998, 6, 983–991. [Google Scholar] [CrossRef]
- Lu, X.; Tang, J.; Cui, S.; Wan, B.; Franzblauc, S.G.; Zhang, T.; Zhang, X.; Ding, K. Pyrazolo[1,5-a]pyridine-3-carboxamide hybrids: Design, synthesis and evaluation of anti-tubercular activity. Eur. J. Med. Chem. 2017, 125, 41–48. [Google Scholar] [CrossRef]
- Kim, S.H.; Lee, Y.H.; Jung, S.Y.; Kim, H.J.; Jin, C.; Lee, Y.S. Synthesis of chromone carboxamide derivatives with antioxidative and calpain inhibitory properties. Eur. J. Med. Chem. 2011, 46, 1721–1728. [Google Scholar] [CrossRef]
- Bylov, I.E.; Vasylyev, M.V.; Bilokin, Y.V. Synthesis and anti-inflammatory activity of N-substituted 2-oxo-2H-1-benzopyran-3-carboxamides and their 2-iminoanalogues. Eur. J. Med. Chem. 1999, 34, 997–1001. [Google Scholar] [CrossRef]
- Venkateswarlu, Y.; Ramesh Kumar, S.; Leelavathi, P. Facile and efficient one-pot synthesis of benzimidazoles using lanthanum chloride. Org. Med. Chem. Lett. 2013, 3, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Foals, D.; Li, J.; Yu, L.; Baldino, C.M. A versatile method for the synthesis of benzimidazoles from o-Nitroanilines and aldehydes in one step via a reductive cyclization. Synthesis 2005, 36, 47–56. [Google Scholar]
- Kumar, V.; Poojary, B.; Prathibha, A.; Shruthi, N. A Synthesis of some novel 1,2-disubstituted benzimidazole-5-carboxylates via One-pot method using Sodium Dithionite and its effect on N-Debenzylation. Synth. Commun. 2014, 44, 3414–3425. [Google Scholar] [CrossRef]
- Roy, P.; Pramanik, A. One-pot sequential synthesis of 1,2-disubstituted benzimidazoles under metal-free conditions. Tetrahedron Lett. 2013, 54, 5243–5245. [Google Scholar] [CrossRef]
- Oda, S.; Shimizu, H.; Aoyama, Y.; Ueki, T.; Shimizu, S.; Osato, H.; Takeuchi, Y. Development of safe one-pot synthesis of N-1- and C-2-Substituted benzimidazole via reductive cyclization of o-Nitroaryl amine using Na2S2O4. Org. Process Res. Dev. 2012, 16, 96–101. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).