Digyaindoleacid A: 2-(1-(4-Hydroxyphenyl)-3-oxobut-1-en-2-yloxy)-3-(1H-indol-3-yl)propanoic Acid, a Novel Indole Alkaloid
Abstract
1. Introduction
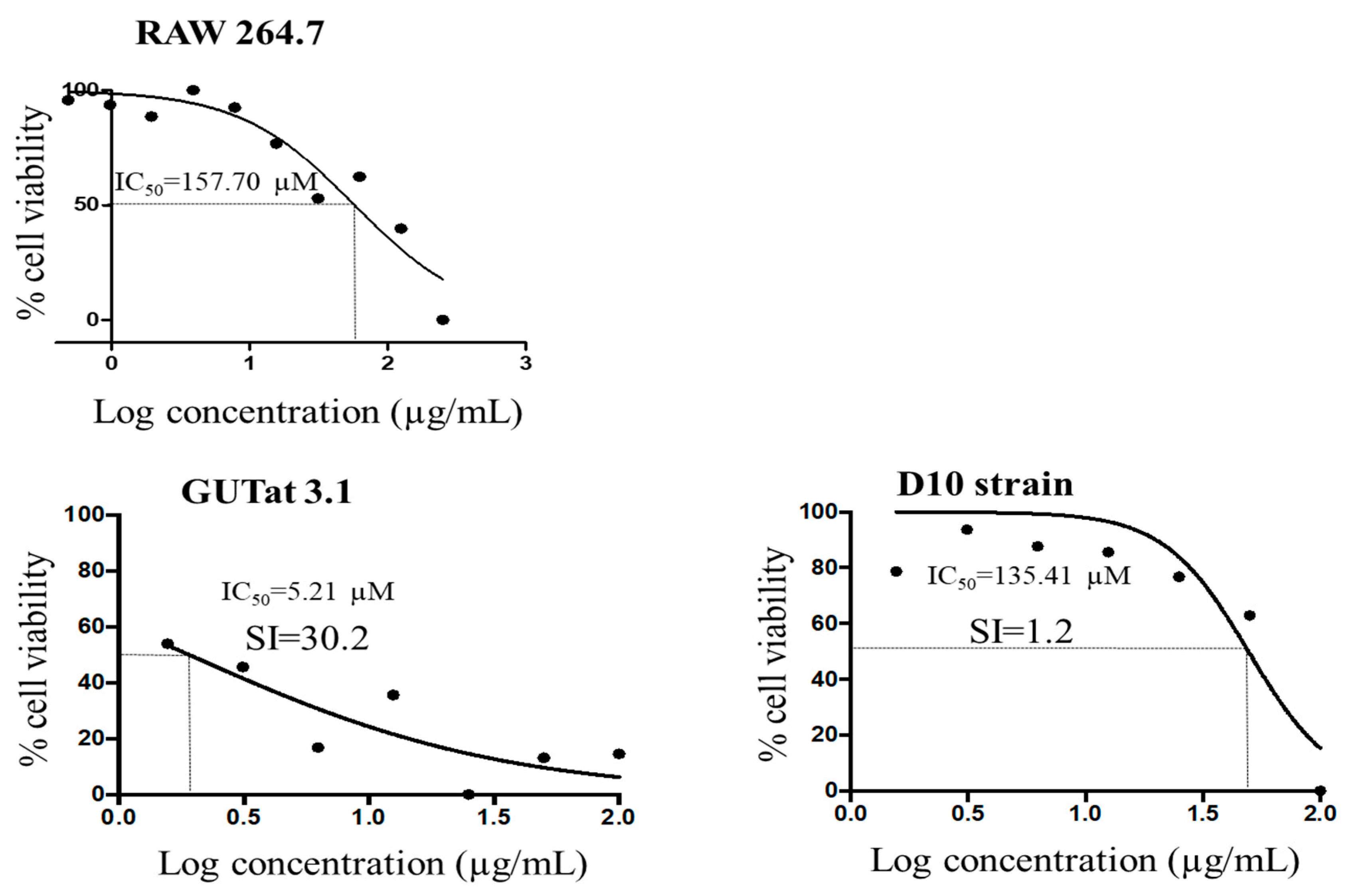
2. Results
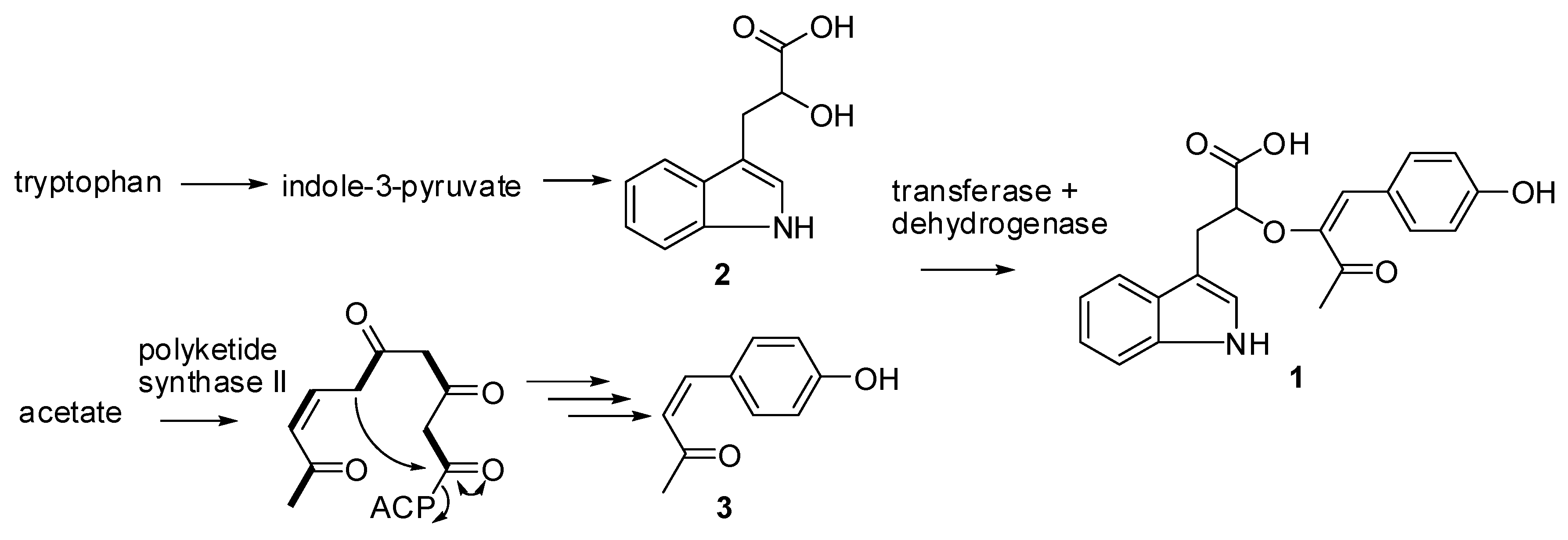
Biosynthesis of Digyaindoleacid A (1)
3. Experimental Section
3.1. General Experimental Procedures
3.2. Sediment Sample Collection Sites
3.3. Isolation, Purification and Taxonomy of Strain DE2SH
3.4. Fermentation
3.5. Extraction, Purification and Isolation
3.6. 2-(1-(4-hydroxyphenyl)-3-oxobut-1-en-2-yloxy)-3-(1H-indol-3-yl) Propanoic Acid (1)
3.7. Culture of Parasites and Mammalian Cell Lines
3.8. Analysis of Cell Viability
3.9. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Standley, C.; Boyce, M.R.; Klineberg, A.; Essix, G.; Katz, R. Organization of oversight for integrated control of neglected tropical diseases within Ministries of Health. PLoS Neglected Trop. Dis. 2018, 12, e0006929. [Google Scholar] [CrossRef] [PubMed]
- Simpson, H.; Quao, B.; Van Der Grinten, E.; Saunderson, P.; Ampadu, E.; Kwakye-MaClean, C.; Odoom, S.; Biritwum, N.-K.; Pullan, R.; Cano, J. Routine surveillance data as a resource for planning integration of NTD case management. Lepr. Rev. 2018, 89, 178–196. [Google Scholar]
- Mitra, A.K.; Mawson, A.R. Neglected Tropical Diseases: Epidemiology and Global Burden. Trop. Med. Infect. Dis. 2017, 2, 36. [Google Scholar] [CrossRef] [PubMed]
- Kirigia, J.M.; Mburugu, G.N. The monetary value of human lives lost due to neglected tropical diseases in Africa. Infect. Dis. Poverty 2017, 6, 165. [Google Scholar] [CrossRef] [PubMed]
- Mathers, C.; Stevens, G.; Hogan, D.; Mahanani, W.R.; Ho, J. Global and regional causes of death: Patterns and trends, 2000–2015. In Disease Control Priorities: Improving Health and Reducing Poverty, 3rd ed.; Jamison, D.T., Gelband, H., Horton, S., Jha, P., Laxminarayan, R., Mock, C.N., Nugent, R., Eds.; The International Bank for Reconstruction and Development/The World Bank: Washington, DC, USA, 2017; Volume 9, pp. 1–429. [Google Scholar]
- Roth, G.A.; Abate, D.; Abate, K.H.; Abay, S.M.; Abbafati, C.; Abbasi, N.; Abbastabar, H.; Abd-Allah, F.; Abdela, J.; Abdelalim, A.; et al. Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1736–1788. [Google Scholar] [CrossRef]
- McDonald, M. Neglected tropical and zoonotic diseases and their impact on women’s and children’s health. In The Causes and Impacts of Neglected Tropical and Zoonotic Diseases: Opportunities for Integrated Intervention Strategies; National Academies Press: Washington, DC, USA, 2011; pp. 357–388. [Google Scholar]
- Mehta, P.; Hotez, P.J. NTD and NCD Co-morbidities: The Example of Dengue Fever. PLoS Negl. Trop. Dis. 2016, 10, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Bhatnagar, T.; Kaur, P.; Kumaraswami, V. Links Between the Epidemiology and Control of Non-communicable Diseases and Neglected Tropical Diseases in Asia. In Neglected Tropical Diseases-East Asia; Utzinger, J., Yap, P., Bratschi, M., Steinmann, P., Eds.; Springer: New York, NY, USA, 2019; pp. 149–173. [Google Scholar]
- Weng, H.-B.; Chen, H.-X.; Wang, M.-W. Innovation in neglected tropical disease drug discovery and development. Infect. Dis. Poverty 2018, 7, 67. [Google Scholar] [CrossRef] [PubMed]
- Akinsolu, F.T.; Nemieboka, P.O.; Njuguna, D.W.; Ahadji, M.N.; Dezso, D.; Varga, O. Emerging Resistance of Neglected Tropical Diseases: A Scoping Review of the Literature. Int. J. Environ. Res. Public Health 2019, 16, 1925. [Google Scholar] [CrossRef] [PubMed]
- Kerr, K.W.; Henry, T.C.; Miller, K.L. Is the priority review voucher program stimulating new drug development for tropical diseases? PLoS Neglected Trop. Dis. 2018, 12, e0006695. [Google Scholar] [CrossRef] [PubMed]
- Osei, E.; Kwain, S.; Mawuli, G.; Anang, A.; Owusu, K.; Camas, M.; Camas, A.S.; Ohashi, M.; Alexandru-Crivac, C.-N.; Deng, H.; et al. Paenidigyamycin A, Potent Antiparasitic Imidazole Alkaloid from the Ghanaian Paenibacillus sp. DE2SH. Mar. Drugs 2019, 17, 9. [Google Scholar] [CrossRef] [PubMed]
- Dofuor, A.K.; Kwain, S.; Osei; Tetevi, G.M.; Okine, L.K.; Ohashi, M.; Gwira, T.M.; Kyeremeh, K.; Osei, E. N-(Isobutyl)-3,4-methylenedioxy Cinnamoyl Amide. Molbank 2019, 2019, M1070. [Google Scholar] [CrossRef]
- Azerigyik, F.A.; Amoa-Bosompem, M.; Tetteh, T.; Ayertey, F.; Antwi, A.N.; Owusu, K.B.-A.; Dadzie, K.K.; Djameh, G.I.; Tetteh-Tsifoanya, M.; Iwanaga, S.; et al. In vitro Mechanistic Assays of Tetracyclic Iridoid Compounds Isolated from Morinda lucida Benth in Leishmania species. Eur. J. Med. Plants 2018, 25, 1–14. [Google Scholar] [CrossRef]
- Yabu, Y.; Minagawa, N.; Kita, K.; Nagai, K.; Honma, M.; Sakajo, S.; Koide, T.; Ohta, N.; Yoshimoto, A. Oral and intraperitoneal treatment of Trypanosoma brucei brucei with a combination of ascofuranone and glycerol in mice. Parasitol. Int. 1998, 47, 131–137. [Google Scholar] [CrossRef]
- Gasparian, A.V.; Burkhart, C.A.; Purmal, A.A.; Brodsky, L.; Pal, M.; Saranadasa, M.; Bosykh, D.A.; Commane, M.; Guryanova, O.A.; Pal, S.; et al. Curaxins: Anticancer Compounds that Simultaneously Suppress NF-κB and Activate p53 by Targeting FACT. Sci. Transl. Med. 2011, 3, 95ra74. [Google Scholar] [CrossRef] [PubMed]
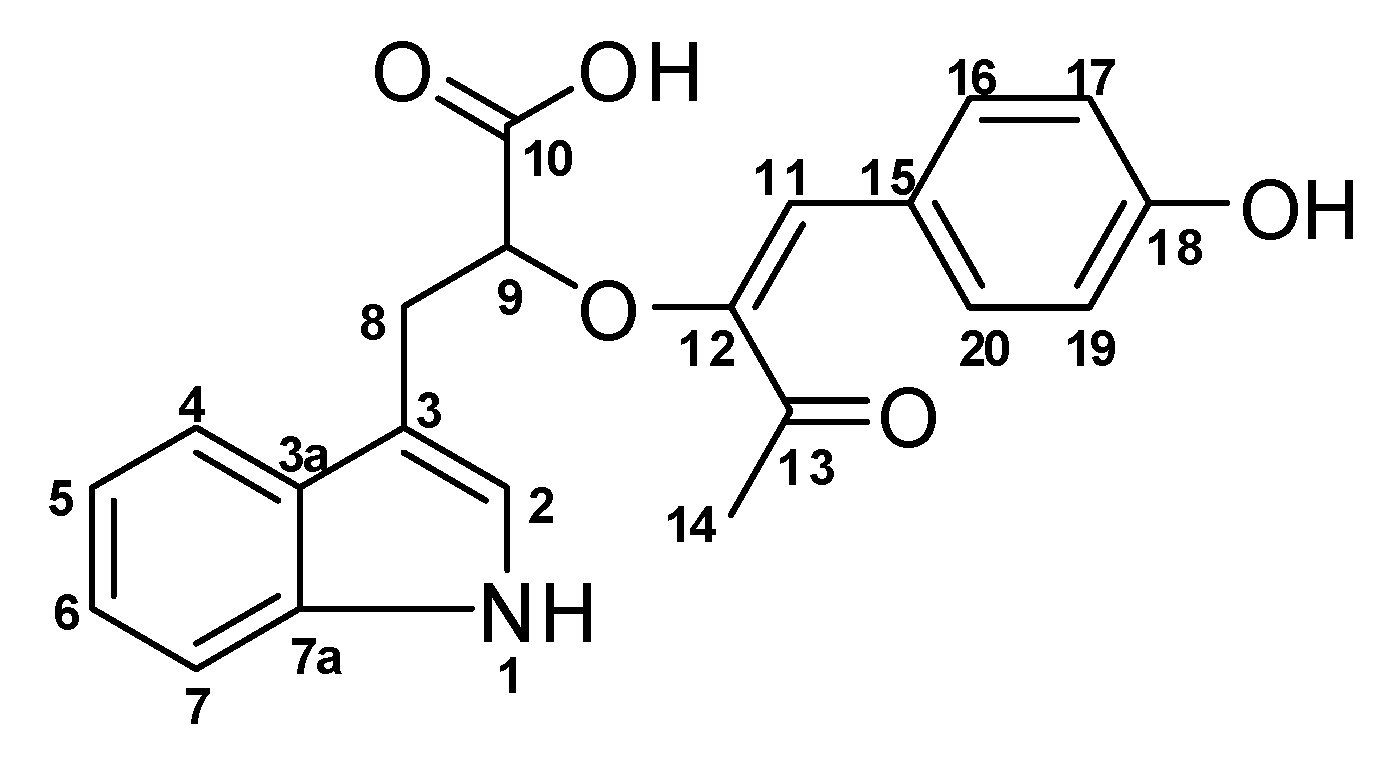
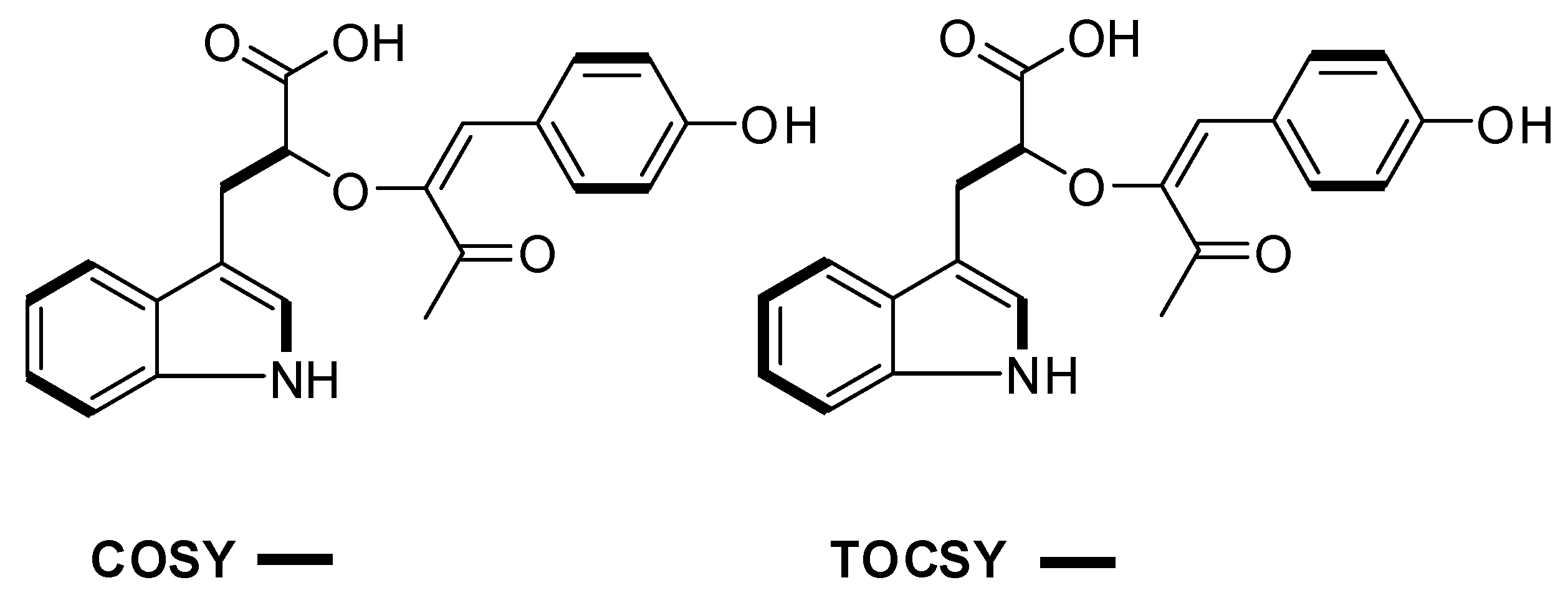
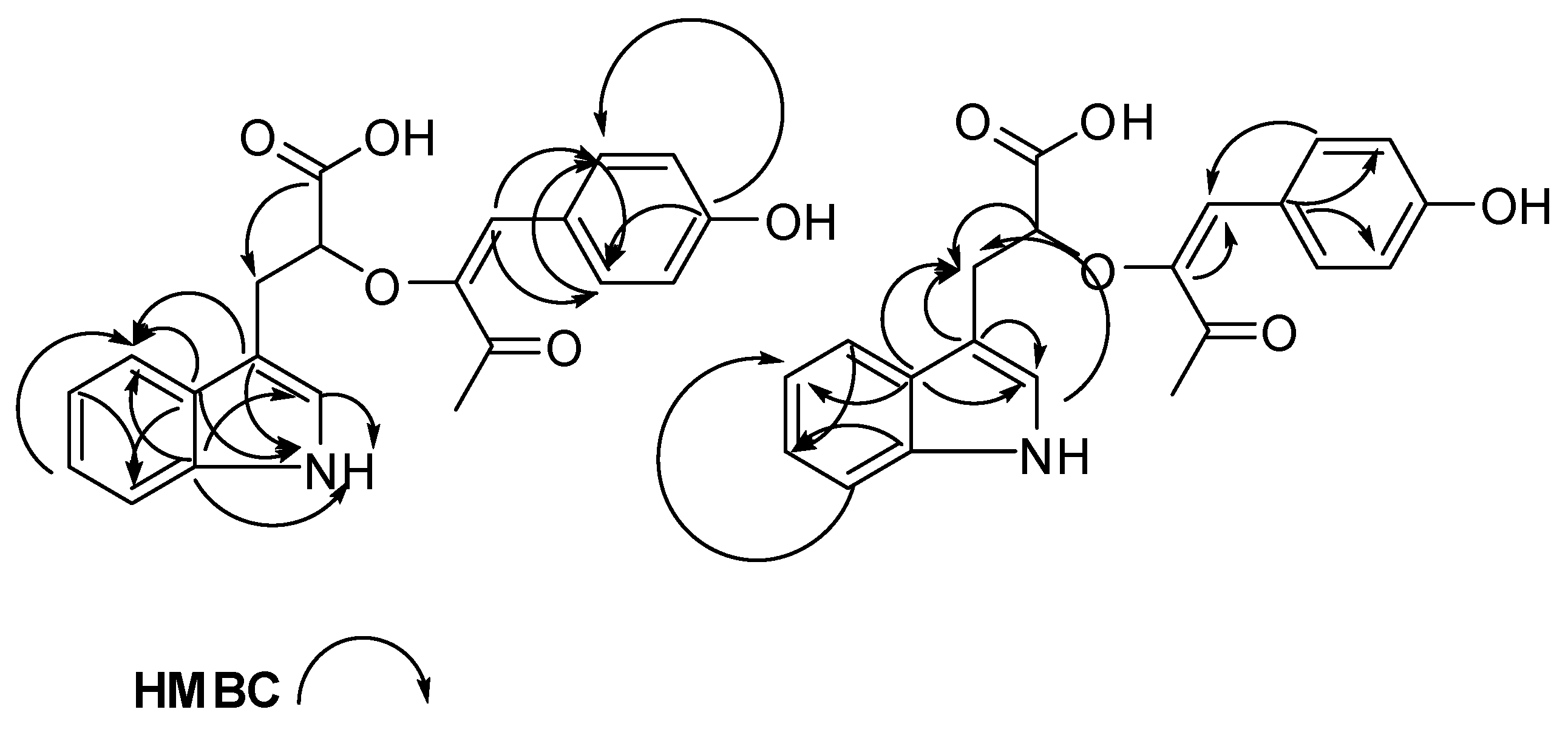
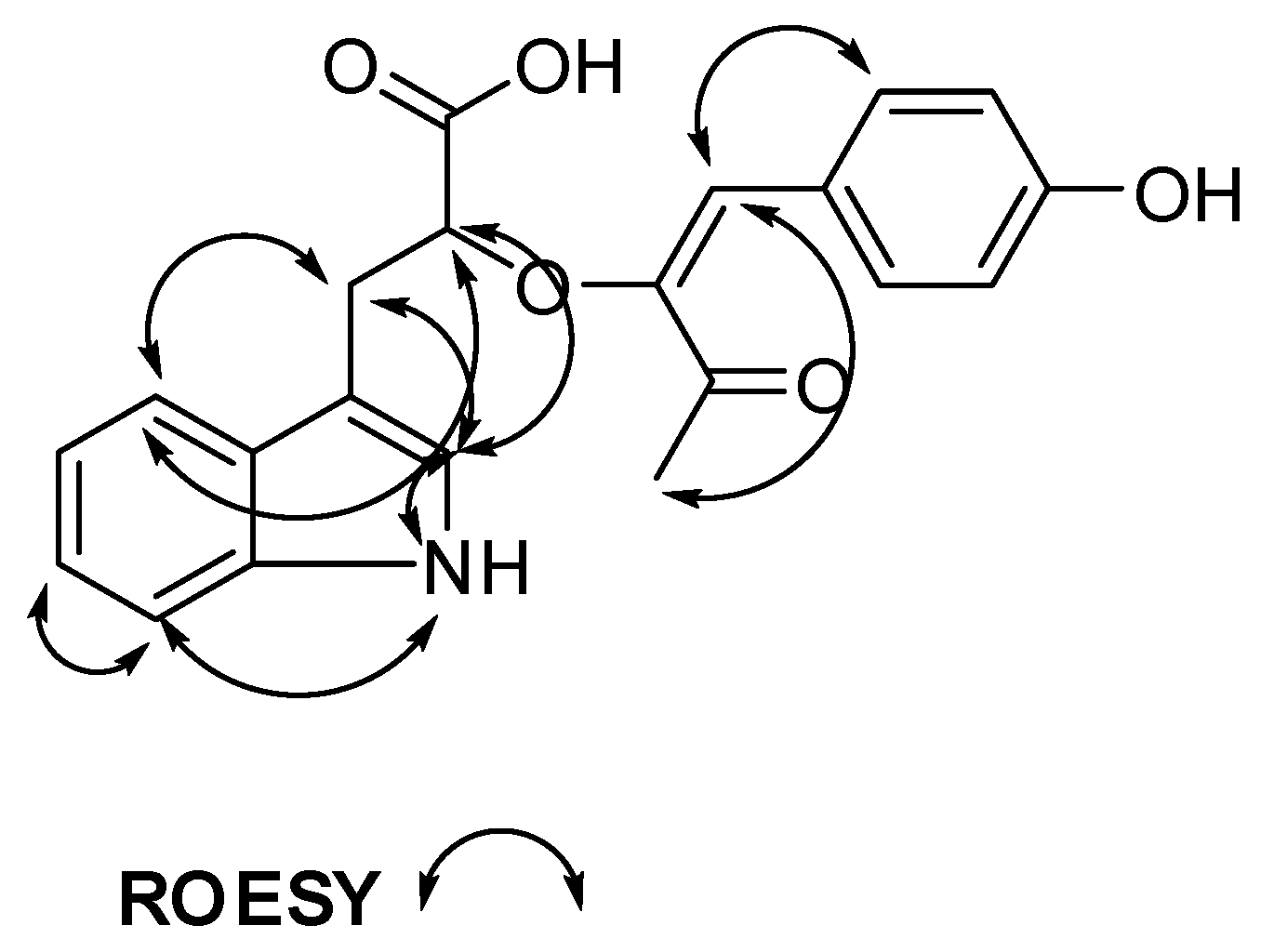
| # | δC mult | δH mult (J Hz) | 1H-1H COSY | HMBC | TOCSY |
|---|---|---|---|---|---|
| 1 NH | 10.67, s | 2 | C-2, C-3, C-3a, C-7a | ||
| 2 | 123.1, CH | 7.10, s | 1NH | C-3, C-3a, C-7a | 1NH |
| 3 | 111.8, C | ||||
| 3a | 127.7, C | ||||
| 4 | 118.6, CH | 7.53, d (7.9) | 5 | C-3, C-3a, C-6, C-7a | 7, 6, 5 |
| 5 | 117.8, CH | 6.93, t (7.9) | 4 | C-3a, C-7 | 4 |
| 6 | 120.4, CH | 7.02, t (7.9) | 7 | C-4, C-7a | 4, 7, 5 |
| 7 | 110.9, CH | 7.30, d (7.9) | 6 | C-3a, C-5 | 4, 6, 5 |
| 7a | 135.8, C | ||||
| 8 | 30.4, CH2 | 3.10, m 2.73, dd (14.5, 8.4) | 9 | C-2, C-3, C-3a, C-9, C-10 | 9 |
| 9 | 71.4, CH | 3.92, m | 8 | 8 | |
| 10 | 176.0, C | ||||
| 10 OH | 4.15, br | ||||
| 11 | 114.3, CH | 6.53, s | C-12, C-16, C-20, C-13 | ||
| 12 | 146.3, C | ||||
| 13 | 194.7, C | ||||
| 14 | 23.8, CH3 | 2.38, s | C-13 | ||
| 15 | 125.7, C | ||||
| 16 | 131.5, CH | 7.69, d (8.5) | 17 | C-11, C-20, C-18 | 17 |
| 17 | 115.3, CH | 6.77, d (8.4) | 16 | C-15 | 16 |
| 18 | 157.4, C | ||||
| 19 | 115.3, CH | 6.77, d (8.4) | 20 | C-15 | 20 |
| 20 | 131.5, CH | 7.69, d (8.5) | 19 | C-11, C-16, C-18 | 19 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kwain, S.; Tetevi, G.M.; Mensah, T.; Camas, A.S.; Camas, M.; Dofuor, A.K.; Azerigyik, F.A.; Deng, H.; Jaspars, M.; Kyeremeh, K. Digyaindoleacid A: 2-(1-(4-Hydroxyphenyl)-3-oxobut-1-en-2-yloxy)-3-(1H-indol-3-yl)propanoic Acid, a Novel Indole Alkaloid. Molbank 2019, 2019, M1080. https://doi.org/10.3390/M1080
Kwain S, Tetevi GM, Mensah T, Camas AS, Camas M, Dofuor AK, Azerigyik FA, Deng H, Jaspars M, Kyeremeh K. Digyaindoleacid A: 2-(1-(4-Hydroxyphenyl)-3-oxobut-1-en-2-yloxy)-3-(1H-indol-3-yl)propanoic Acid, a Novel Indole Alkaloid. Molbank. 2019; 2019(3):M1080. https://doi.org/10.3390/M1080
Chicago/Turabian StyleKwain, Samuel, Gilbert Mawuli Tetevi, Thomas Mensah, Anil Sazak Camas, Mustafa Camas, Aboagye Kwarteng Dofuor, Faustus Akankperiwen Azerigyik, Hai Deng, Marcel Jaspars, and Kwaku Kyeremeh. 2019. "Digyaindoleacid A: 2-(1-(4-Hydroxyphenyl)-3-oxobut-1-en-2-yloxy)-3-(1H-indol-3-yl)propanoic Acid, a Novel Indole Alkaloid" Molbank 2019, no. 3: M1080. https://doi.org/10.3390/M1080
APA StyleKwain, S., Tetevi, G. M., Mensah, T., Camas, A. S., Camas, M., Dofuor, A. K., Azerigyik, F. A., Deng, H., Jaspars, M., & Kyeremeh, K. (2019). Digyaindoleacid A: 2-(1-(4-Hydroxyphenyl)-3-oxobut-1-en-2-yloxy)-3-(1H-indol-3-yl)propanoic Acid, a Novel Indole Alkaloid. Molbank, 2019(3), M1080. https://doi.org/10.3390/M1080







