Abstract
The title compound, 6-[1-acetyl-5-(4-methoxyphenyl)-4,5-dihydro-1H-pyrazol-3-yl]-2(3H)-benzoxazolone, was synthesized by condensation of 6-[3-(4-methoxyphenyl)-2-propenoyl]-2(3H)-benzoxazolone (1) and hydrazine hydrate in acetic acid in 84% yield. The structure of the target compound was confirmed using 1H-NMR, 13C-NMR, IR, MS, and elemental analysis.
1. Introduction
Chalcones are important compounds in medicinal chemistry, which exhibit diverse biological activities [1] and undergo a variety of chemical reactions that lead to several heterocyclic systems [2]. Pyrazole derivatives are also known to exhibit antimicrobial, antiviral, and anti-inflammatory activities [3,4,5]. In the present study we use a heterocyclic chalcone as an intermediate for the synthesis of a new compound with a pyrazole moiety.
2. Results
The synthesis of 6-[1-acetyl-5-(4-methoxyphenyl)-4,5-dihydro-1H-pyrazole-3-yl]-2(3H)-benzoxazolone (2) (Scheme 1) was performed by reaction of 6-[3-(4-methoxyphenyl)-2-propenoyl]-2(3H)-benzoxazolone (1) with hydrazine hydrate. The reaction was carried out in acetic acid at 115 °C for 1 h and led to the formation of a single product in good yield.
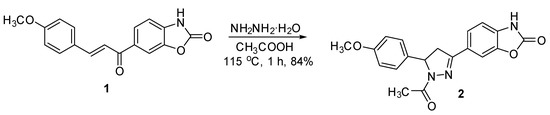
Scheme 1.
Synthesis of 6-[1-acetyl-5-(4-methoxyphenyl)-4,5-dihydro-1H-pyrazole-3-yl]-2(3H)-benzoxazolone (2).
The structure of compound 2 was confirmed by 1H and 13C-NMR, IR, MS and elemental analysis and all data are in accordance with the proposed structure. The C=O stretching bands in IR spectra were seen at about 1610 cm−1 and 1780 cm−1. In the 1H-NMR spectrum the vinyl proton signals characteristic of the starting chalcone 1 are replaced by three multiplets corresponding to the pyrazole protons in the range δ = 3.12–5.48 ppm. Aromatic protons are observed as doublets of doublets at δ = 6.87–7.68 ppm. Three singlet signals corresponding to the methyl, methoxy, and urethane groups are observed at 2.28, 3.71, and 11.9 ppm, respectively. The 13C-NMR spectrum displays 17 signals corresponding to the total number of carbon atoms in compound 2.
3. Experimental Section
3.1. General Information
All chemicals were purchased from Acros Organics (Geel, Belgium). 6-[3-(4-Methoxyphenyl)-2-propenoyl]-2(3H)-benzoxazolone (1) was synthesized as described previously [6,7]. Reactions and purity of the final compound were monitored by thin-layer chromatography (TLC) on silica gel plates (Kieselgel 60 F254), using ethyl acetate/cyclohexane (3:2 v/v) as eluent.
Melting points were determined on a Boetius hot-stage microscope (Carl Zeiss Jena, Germany). IR spectrum (nujol) was recorded on a Specord 71 spectrometer (Carl Zeiss Jena, Germany). NMR spectra were recorded in DMSO-d6 on a Bruker Avance III HD 500 (Bruker BioSpin GmbH, Rheinstetten, Germany), operating at 500 MHz for 1H and at 125.8 MHz for 13C. Chemical shifts are given in parts per million (δ) relative to the solvent peak. Coupling constants (J) were measured in hertz (Hz). Mass spectra were recorded on an Agilent 6890 system (Wilmington, DE, USA) with MSD 5973 (single quadrupol and electron ionization (EI) at 70 eV ionization), using a capillary column HP-5/MS (30 m × 0.250 mm × 0.25 µm). Carrier gas He was used at 0.8 mL/min. The temperature programmed mode was used (from 60 °C for 2 min, then with 10 °C/min to 300 °C for 10 min). The sample was introduced in splitless injection mode. The elemental analysis was carried on a “VARIO EL III Elemental analyzer” (Elementar Analysensysteme GmbH, Hanau, Germany) and the results for C, H, and N were within ±0.4% of the theoretical values.
3.2. Synthesis of 6-[1-Acetyl-5-(4-methoxyphenyl)-4,5-dihydro-1H-pyrazole-3-yl]-2(3H)-benzoxazolone (2)
To a suspension of 6-[3-(4-methoxyphenyl)-2-propenoyl]-2(3H)-benzoxazolone (1, 295 mg, 1 mmol) in acetic acid (10 mL), hydrazine hydrate (200 mg, 4 mmol) was added. The reaction mixture was refluxed for 1 h, until the reaction was complete as monitored by TLC. The resulting yellow solution was poured on crushed ice. The crystalline product was filtered, washed with water, and dried.
Pale orange crystals. Yield: 84% (294 mg), m.p.: 275–277 °C (acetic acid). IR (nujol): 1610, 1780 (C=O) cm−1. 1H-NMR (500 MHz, DMSO-d6): δ (ppm) 2.28 (s, 3H, CH3), 3.12 (dd, 1H, pyrazoline, J = 18.0 Hz, J = 4.4 Hz), 3.71 (s, 3H, CH3), 3.80 (dd, 1H, pyrazoline, J = 18.0 Hz, J = 12.0 Hz), 5.48 (dd, 1H, pyrazoline, J = 12.0 Hz, J = 4.3 Hz), 6.87 (d, 2H, arom. H, J = 8.7 Hz), 7.10 (d, 2H, arom. H, J = 8.7 Hz), 7.15 (d, 1H, arom. H, J = 8.1 Hz), 7.58 (dd, 1H, arom. H, J = 8.1 Hz, J = 1.5 Hz), 7.68 (d, 1H, arom. H, J = 1.3 Hz), 11.9 (brs, 1H, NH). 13C-NMR (125.8 MHz, DMSO-d6): δ (ppm) 21.8, 42.2, 55.1, 59.0, 107.4, 109.8, 114.0, 123.0, 125.3, 126.8, 132.3, 134.5, 143.6, 153.9, 154.4, 158.4, 167.2. Anal. calcd. for C19H17N3O4 (351.36): C, 64.95; H, 4.88; N 11.96. Found: C, 64.91; H, 4.79; N, 11.72. MS (EI): [M]+ m/z = 351(56), [M + 2]+ m/z = 353(3), 308(100), 294(8), 278(9), 264(6), 202(17), 191(12), 176(25), 161(4), 149(9), 134(15), 121(8).
Supplementary Materials
The following are available online, 1H-, 13C-NMR and MS spectra for compound 2.
Author Contributions
O.P. and Y.I. designed the experiments; Y.I. and A.T. performed the experiments; O.P. and Y.I. analyzed the spectral data and wrote the manuscript; C.C. obtained the mass spectra. All authors read and approved the final manuscript.
Funding
This research was funded by University of Forestry, Sofia, Bulgaria, grant number B30/07.03.2018 and Bulgarian Science Fund, grant number DN19/13, 2017.
Acknowledgments
The authors are thankful to University of Forestry, Sofia, Bulgaria (Contract B30/07.03.2018) and to Bulgarian Science Fund (Contract DN19/13, 2017) for financial support.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Nowakowska, Z. A review of anti-infective and anti-inflammatory chalcones. Eur. J. Med. Chem. 2007, 42, 125–137. [Google Scholar] [CrossRef] [PubMed]
- Kalirajan, R.; Sivakumar, S.; Jubie, S.; Gowramma, B.; Suresh, B. Synthesis and biological evaluation of some heterocyclic derivatives of chalcones. Int. J. ChemTech Res. 2009, 1, 27–34. [Google Scholar]
- Kumar, V.; Kaur, K.; Gupta, G.; Sharma, A. Pyrazole containing natural products: Synthetic preview and biological significance. Eur. J. Med. Chem. 2013, 69, 735–753. [Google Scholar] [CrossRef] [PubMed]
- Ansari, A.; Ali, A.; Asif, M.; Shamsuzzaman. Review: Biologically active pyrazole derivatives. New J. Chem. 2017, 41, 16–41. [Google Scholar] [CrossRef]
- Rana, D.; Chhabria, M.; Shah, N.; Brahmkshatriya, P. Pharmacophore combination as a useful strategy to discover new antitubercular agent. Med. Chem. Res. 2014, 23, 370–381. [Google Scholar] [CrossRef]
- Ivanova, Y.; Momekov, G.; Petrov, O.; Karaivanova, M.; Kalcheva, V. Cytotoxic Mannich bases of 6-(3-aryl-2-propenoyl)-2(3H)-benzoxazolones. Eur. J. Med. Chem. 2007, 42, 1382–1387. [Google Scholar] [CrossRef] [PubMed]
- Ivanova, Y.; Momekov, G.; Petrov, O. New heterocyclic chalcones. Part 6. Synthesis and cytotoxic activities of 5- or 6-(3-aryl-2-propenoyl)-2(3H)-benzoxazolones. Heterocycl. Commun. 2013, 19, 23–28. [Google Scholar] [CrossRef]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).