Abstract
4-(1H-[1,2,4]-Triazol-5-ylsulfanyl)-1,2-dihydropyrazol-3-one (4) was synthesized with a yield of 55% via ring-switching hydrazinolysis of 5-ethoxymethylidenethiazolo[3,2-b][1,2,4] triazol-6-one (3) in ethanol medium. The initial 1H-[1,2,4]-triazole-3-thiol (1) was modified via a two-step reaction: S-alkylation with chloroacetic acid under Williamson reaction conditions, and further one-pot cyclization–condensation with triethylorthoformate in the acetic anhydride medium, yielding compound 3. The structures of compounds 3 and 4 were confirmed by LC-MS, NMR spectra and a single X-ray diffraction analysis (for compound 4).
1. Introduction
Recyclization and transformation reactions are powerful and useful tools in modern organic and medicinal chemistry for the synthesis of hard-to-reach heterocycles. The compounds with an “enone” system in their structure, such as 3-alkylaminopropen-1-one fragments, combine the ambident nucleophilicity of enamines with the ambident eletrophilicity of enones. Due to these properties, “enones” are often used as C3 building blocks for ring-switching reactions, yielding condensed and non-condensed heterocycles [1,2,3]. However, the synthetic pathways for 3-alkylaminepropene-1-one derivatives may be accompanied by a number of undesirable parallel processes such as methylation, the formation of major side products, and difficulties in isolating the target compounds. At the same time, the modification of 3-alkoxypropene-1-ones as the equivalent of 3-alkylaminepropene-1-one for the construction of heterocycles in the recyclization reactions is undescribed. Therefore, the utilization of 3-alkoxypropene-1-one analogs of corresponding enaminones can be a promising alternative for the preparative organic chemistry of “enones”. To extend our work in the development of effective synthetic methods for biologically active molecules based on functionalized 4-thiazolidinones and thiazolo[3,2-b][1,2,4]triazol-6-one [4,5,6,7], in this paper, we described the synthesis of hard-to-reach 4-(2H-[1,2,4]-triazol-5-ylsulfanyl)-1,2-dihydropyrazol-3-one (4) from the derivative with a 3-alkoxypropene-1-one moiety, namely 5-ethoxymethylidenethiazolo[3,2-b][1,2,4]triazol-6-one (3).
2. Results and Discussion
2.1. Chemistry
1H-[1,2,4]-triazole-3-thiol (1) was used as the starting reagent for the synthesis of the target compounds (Scheme 1). In the first step (2H-[1,2,4]-triazol-3-ylsulfanyl)-acetic acid (2) was synthesized under Williamson reaction conditions from compound 1 via alkylation with chloroacetic acid. Compound 2 was utilized without any purification in reaction with triethylorthoformate in acetic anhydride, yielding 5-ethoxymethylidenethiazolo[3,2-b][1,2,4]triazol-6-one (3). In this case, acetic anhydride plays a dual role as a cyclization agent for the thiazolo[3,2-b][1,2,4]triazol-6-one system formation and as a catalyst for the condensation of a methylene group with triethylorthoformate. Finally, compound 3 was refluxed with hydrazine hydrate in ethanol for 15 min, producing pyrazol-3-one derivative 4 with a 55% yield.
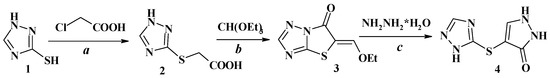
Scheme 1.
Synthesis of 5-ethoxymethylidenethiazolo[3,2-b][1,2,4]triazol-6-one (3) and 4-(1H-[1,2,4]-triazol-5-ylsulfanyl)-1,2-dihydropyrazol-3-one (4). Reagents and conditions: (a) 1 (1.0 eq.), chloroacetic acid (1.0 eq.), AcONa (2.0 eq.), AcOH, reflux, 2 h, 76%; (b) 2 (1.0 eq.), triethylorthoformate (1.25 eq.), Ac2O, reflux, 2 h, 78%; (c) 3 (1.0 eq.), hydrazine hydrate (1.0 eq.), EtOH, reflux, 15 min, 55%.
The structure and purity of compounds 3 and 4 were confirmed by LC-MS, 1H and 13C NMR spectra (copies of spectra are presented in Supplementary). In the 1H NMR spectra of compounds 3 and 4, protons of the triazol ring appeared as singlets at 8.50 (C-3) and 8.26 ppm (C-2), respectively. A chemical shift in the methylidene group of compound 3 has been assigned in a weak magnetic field at 8.41 ppm. A signal of the pyrazole ring proton at C-5 of compound 4 was observed as a singlet at 7.71 ppm.
2.2. X-ray Crystallographic Analysis of the Compound 4
X-ray analysis was used for the structure determination of compound 4. The ORTEP drawing and atomic numbering are shown in Figure 1. The performed X-ray studies revealed that the 1,2,4-triazole system in the molecule of compound 4 undergoes a tautomeric transition in a solution. The crystals 4(A) (lath shape) and 4(B) (prism shape), obtained simultaneously from the same solution of CH3OH and H2O mixed at a 1:1 ratio, prove this conclusion. The lath-shaped crystals, 4(A), have the tautomeric hydrogen atoms at N2′ while the prism-shaped ones, 4(B), have these hydrogen atoms located at N1 (Figure 1).

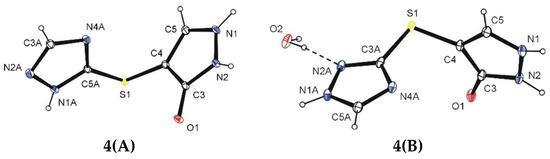
Figure 1.
The molecular structures of compound 4 in the crystals 4(A) and 4(B).
The X-ray studies has shown that proton tautomerism is observed in the 1,2,4-triazole system. In the first case (crystal 4(A)), the 1,2,4-triazole system has a tautomeric structure with hydrogen located at N1A and 4-sulfanyl-1,2-dihydro-3H-pyrazol-3-one moiety located at the C5 atom (form A) while in the other one (crystal 4(B)), the hydrogen atom is located at N1A and the 4-sulfanyl-1,2-dihydro-3H-pyrazol-3-one moiety at the C3 atom (form B) (Scheme 2).
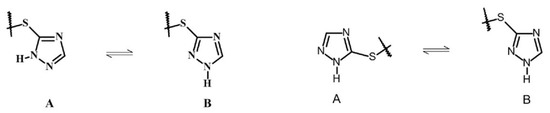
Scheme 2.
Possible tautomeric forms of the 1,2,4-triazole moiety of compound 4.
The positions of protons connected to N atoms in both heterocyclic systems of molecules in crystals 4(A) and 4(B), i.e., 1,2-dihydro-3H-pyrazol-3-one and 1,2,4-triazole systems, were obtained from the difference Fourier maps and were refined freely. The presence of the H atom in the 1,3,4-triazole system at N1A (the molecules in the crystal 4(A)) and at N1A (the molecules in the crystal 4(B)) is supported by intermolecular hydrogen bonds N1A–H1A∙∙∙O1ii (Table 1, Figure 2) and N1A–H1A∙∙∙O1ii (Table 1, Figure 3). In the mentioned bonds, the oxygen atom from the carbonyl group (O1) acts as a proton acceptor.

Table 1.
Hydrogen-bond geometry (Å, °) for 4(A) and 4(B).
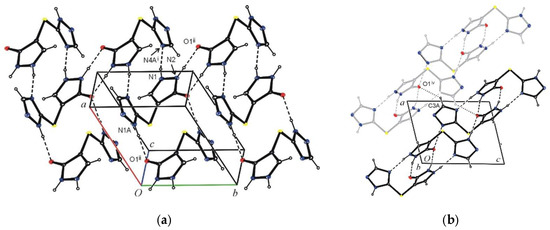
Figure 2.
Part of the molecular packing in the crystal 4(A), showing (a) molecules linked by N–H∙∙∙N, N–H∙∙∙O hydrogen bonds and (b) the hydrogen bonded (C–H∙∙∙O) sheets parallel to the (10-1) plane. The H atoms not involved in hydrogen bonds have been omitted for clarity.
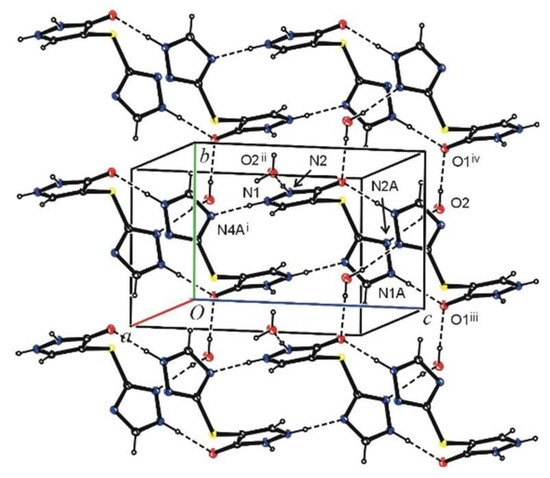
Figure 3.
The hydrogen bonding in the crystal 4(B). The H atoms not involved in hydrogen bonds have been omitted for clarity.
The molecules of the investigated compound 4 in crystals 4(A) and 4(B) display similar conformations. The dihedral angle between the heterocyclic rings is 89.51(6)° and 89.02(7)° in crystals 4(A) and 4(B), respectively. Moreover, the torsion angles of C4–S1–C5A–N1A (crystal 4(A)) and C4–S1–C3A–N2A (crystal 4(B)) adopt values of −152.08(13)° and −159.47(14)°, respectively. The study revealed that the C3=O1 bond lengths in 4a and 4b are 1.2763(19) and 1.273(2)°, respectively, which indicates a significant elongation with respect to the value in the literature of the (C*)2-C=O bond length 1.210(1) Å [8]. On the other hand, the observed values are typical when compared to C=O bonds in the structures with 1,2-dihydro-3H-pyrazol-3-one system deposited in Cambridge Structural Database, Version 5.39 (refcodes: CASJIQ, CIHNAI, HIFXOJ, HMBINZ, HUFWUB, IKABAY, IKALIQ, MPYAZO10, MPYAZO11, PIDSEB, QAKJUJ, TUPHAO, VEYVEA, VUZQEN) [9].
In crystal 4(A), the molecules are connected by N1–H1∙∙∙N4Ai and N2–H2∙∙∙O1iii hydrogen bonds into columns extending along the [101] direction. The columns are further connected by N1A–H1A∙∙∙O1ii hydrogen bonds into layers parallel to (101) plane. The layers linked with C3A–H3A∙∙∙O1iv hydrogen bonds form three-dimensional network (Table 1, Figure 2a,b). Crystal 4(B) has a form of a hydrate. The asymmetric unit contains one solute molecule (host) and one water molecule (guest). In the crystal solute, molecules are connected by N1–H1∙∙∙N4Ai and N1A–H1A∙∙∙O1ii hydrogen bonds into columns as in crystal 4(A), but in this case, they extend along the c axis. Water molecules are also involved in the hydrogen bond formation. They act as a proton donor twice and as a proton acceptor once. Taking part in N2–H2∙∙∙O2iii, O2–H21∙∙∙N2A and O2–H22∙∙∙O1iv hydrogen bonds, water molecules are a link connecting the mentioned columns into a three-dimensional network (Table 1, Figure 3).
3. Experimental Setup
3.1. Materials and Methods
Melting points were measured in open capillary tubes on a BŰCHI B-545 melting point apparatus (BÜCHI Labortechnik AG, Flawil, Switzerland) and are uncorrected. The elemental analyses (C, H, N) were performed using the Perkin–Elmer 2400 CHN analyzer (PerkinElmer, Waltham, MA, USA) and were within ±0.4% of the theoretical values. The 1H and 13C NMR spectra were recorded on a Varian Gemini spectrometer (Agilent, Santa Clara, CA, USA) at 400 and 100 MHz, respectively, using DMSO-d6 as a solvent and TMS as an internal standard. Chemical shift values are reported in ppm units with use of δ scale. LC–MS spectra were obtained on a Finnigan MAT INCOS-50 (Thermo Finnigan LLC, San Jose, CA, USA). Solvents and reagents that are commercially available were used without further purification. Compound 3 (CAS 2111914-32-8) is also commercially available from Ambinter [10]; Aurora Fine Chemicals [11]; Zerenex Molecular Ltd. [12] and compound 4 (CAS 2176338-67-1) is also commercially available from Aurora Fine Chemicals [11] although it is rather expensive.
3.2. Synthesis of 5-ethoxymethylidenethiazolo[3,2-b][1,2,4]triazol-6-one (3)
A mixture of (2H-[1,2,4]triazol-3-yflsulfanyl)-acetic acid (2) (10.0 mmol) and triethylorthoformate (12.5 mmol) refluxed for 2 h in the 10 mL of acetic anhydride. Reaction mixture was cooled to the room temperature and light brown precipitate was filtered off, washed with small portion of cold water and recrystallized from ethanol.
Light brown needle-like crystals had the following yields: 78%, M.p.: 169–173 °C. 1Н NMR (400 MHz, DMSO-d6), δ: 8.50 (s, 1Н, СН, triazole), 8.41 (s, 1Н, СН), 4.47 (q, J = 6.9 Hz, 2Н, OСН2), 1.32 (t, J = 6.9 Hz, 3Н, СН3). 13C NMR (100 MHz, DMSO-d6), δ: 162.4, 159.5, 158.7, 156.8, 104.0, 73.7, 15.7. LC-MS: m/z 198 (100%, (M + H)+). Anal. Calcd for C7H7N3O2S (197): C 42.63, H 3.58, N 21.31. Found: C 42.40, H 3.50, N 21.50.
3.3. Synthesis of 4-(2H-[1,2,4]-triazol-5-ylsulfanyl)-1,2-dihydropyrazol-3-one (4)
A mixture of 5-ethoxymethylidenethiazolo[3,2-b][1,2,4]triazol-6-one (3) (10.0 mmol) and hydrazine hydrate (10.0 mmol) were refluxed for 15 min in 10 mL of ethanol. After cooling to room temperature, white precipitate was filtered off, washed with ethanol, recrystallized from a mixture of DMFA–ethanol (1:1) and dried under vacuum.
White crystals had the following yields: 55%, M.p.: 223–225 °C. 1Н NMR (400 MHz, DMSO-d6), δ: 8.26 (br.s, 1Н, СН, triazole), 7.71 (s, 1Н, СН, pyrazol). 13C NMR (100 MHz, DMSO-d6), δ: 167.4, 161.8, 157.8, 136.1, 101.6. LC-MS: m/z 184 (100%, (M + H)+). Anal. Calcd for C5H5N5OS (183): C 32.78, H 2.75, N 38.23. Found: C 32.90, H 2.90, N 38.40.
3.4. X-ray Diffraction Studies
3.4.1. Crystal Structure Determination of 4(A)
Crystal data. C5H5N5OS, Mr = 183.20, triclinic, space group P-1, a = 6.8457(4), b = 6.9769(5), c = 8.4306(5) Å, α = 105.028(5), β = 96.908(5), γ = 111.870(6)°, V = 350.18(4) Å3, T = 130.1(1) K, Z = 2.
Data collection. A colourless prism (MeOH/H2O) crystal of 0.13 mm × 0.13 mm × 0.03 mm was used to record 5773 (Cu Kα radiation, θmax = 76.42°) intensities on a Rigaku SuperNova Dual Atlas diffractometer [13] using mirror monochromatized Cu Kα radiation from a high-flux microfocus source. Accurate unit cell parameters were determined by least-squares techniques from the θ values of 3460 reflections, θ range 5.56–76.32°. The data were corrected for Lorentz polarization and for absorption effects [13]. The 1460 total unique reflections (Rint = 0.026) were used for structure determination.
Structure solution and refinement. The structure was solved by a dual-space algorithm (SHELXT) [14], and refined against F2 for all data (SHELXL-97) [15]. The positions of all H atoms were obtained from the difference Fourier maps and were refined freely. Final refinement converged with R = 0.029 (for 1392 data with F2 > 4σ(F2)), wR = 0.078 (on F2 for all data), and S = 1.048 (on F2 for all data). The largest difference peak and hole was 0.325 and −0.325 eÅ3. The molecular illustrations were drawn using ORTEP-3 for Windows [16].
The crystallographic data in the CIF form are available as Electronic Supplementary data from the Cambridge Crystallographic Data Centre, deposition number CCDC-1859739; http://www.ccdc.cam.ac.uk/conts/retrieving.html (or from the Cambridge Crystallographic Data Centre, 12 Union Road, Cambridge, CB2 1EZ, UK; fax: 44 1223 336033; e-mail: deposit@ccdc.cam.uk).
3.4.2. Crystal Structure Determination of 4(B)
Crystal data. C5H5N5OS·H2O, Mr = 201.22, triclinic, space group P-1, a = 6.3934(4), b = 6.7817(5), c = 10.0195(7) Å, α = 92.181(6), β = 107.435(6), γ = 102.234(6)°, V = 402.65(5) Å3, T = 130.1(1) K, Z = 2.
Data collection. A colourless lath (MeOH/H2O) crystal of 0.22 mm × 0.15 mm × 0.03 mm was used to record 6319 (Cu Kα radiation, θmax = 76.75°) intensities on a Rigaku SuperNova Dual Atlas diffractometer [13] using mirror monochromatized Cu Kα radiation from a high-flux microfocus source. Accurate unit cell parameters were determined by least-squares techniques from the θ values of 2958 reflections, θ range 7.42–75.94°. The data were corrected for Lorentz polarization and for absorption effects [13]. The 1646 total unique reflections (Rint = 0.050) were used for structure determination.
Structure solution and refinement. The structure was solved by dual-space algorithm (SHELXT) [14], and refined against F2 for all data (SHELXL-97) [15]. The positions of all H atoms obtained from the difference Fourier maps and were refined freely. Final refinement converged with R = 0.035 (for 1519 data with F2 > 4σ(F2)), wR = 0.096 (on F2 for all data), and S = 1.066 (on F2 for all data). The largest difference peak and hole was 0.494 and −0.399 eÅ3. The molecular illustrations were drawn using ORTEP-3 for Windows [16].
The crystallographic data in the CIF form are available as Electronic Supplementary data from the Cambridge Crystallographic Data Centre, deposition number CCDC-1859740; http://www.ccdc.cam.ac.uk/conts/retrieving.html (or from the Cambridge Crystallographic Data Centre, 12 Union Road, Cambridge, CB2 1EZ, UK; fax: 44 1223 336033; e-mail: deposit@ccdc.cam.uk).
4. Conclusions
We have described a simply executable synthetic method for 4-(2H-[1,2,4]triazol-5-ylsulfanyl)-1,2-dihydropyrazol-3-one via ring-switching hydrazinolysis of 5-ethoxymethylidenethiazolo[3,2-b][1,2,4]triazol-6-one. All studied reactions are convenient due to their conditions, short time, and simple working procedures and allowed us to obtain the target products with high yields.
Supplementary Materials
The following are available online, Figure S1: LC-MS spectrum of compound 3, Figure S2: 1H NMR spectrum of compound 3, Figure S3: 13C NMR spectrum of compound 3, Figure S4: LC-MS spectrum of compound 4, Figure S5: 1H NMR spectrum of compound 4, Figure S6: 13C NMR spectrum of compound 4.
Author Contributions
Conceptualization, R.L. and S.H.; Synthesis of Target Compounds, S.H., H.D. and Y.S.; Spectral Data, O.K.; X-ray Analysis, A.G.; Writing-Original Draft Preparation, S.H.; Writing-Review & Editing, R.L.; Supervision, R.L. All authors read and approved the final manuscript.
Funding
The project was partly supported by State Fund for Fundamental Research of Ukraine (F76/72-2017) and Ministry of Education and Science of Ukraine (M/181-2017, Ukrainian-Austrian project).
Acknowledgments
This research was supported by the Danylo Halytsky Lviv National Medical University, which is gratefully acknowledged.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Stanovnik, B.; Svete, J. Synthesis of Heterocycles from Alkyl 3-(Dimethylamino)propenoates and Related Enaminones. Chem. Rev. 2004, 104, 2433–2480. [Google Scholar] [CrossRef] [PubMed]
- Svete, J. Utilisation of chiral enaminones and azomethine imines in the synthesis of functionalized pyrazoles. ARKIVOC 2006, 7, 35–56. Available online: https://www.arkat-usa.org/get-file/23239/ (accessed on 8 August 2018).
- Negri, G.; Kascheres, C.; Kascheres, A.J. Recent Development in Preparation Reactivity and Biological Activity of Enaminoketones and Enaminothiones and Their Utilization to Prepare Heterocyclic Compounds. J. Heterocycl. Chem. 2004, 41, 461–491. [Google Scholar] [CrossRef]
- Kaminskyy, D.; Kryshchyshyn, A.; Lesyk, R. 5-Ene-4-thiazolidinones—An efficient tool in medicinal chemistry. Eur. J. Med. Chem. 2017, 140, 542–594. [Google Scholar] [CrossRef] [PubMed]
- Kaminskyy, D.; Kryshchyshyn, A.; Nektegayev, I.; Vasylenko, O.; Grellier, P.; Lesyk, R. Isothiocoumarin-3-carboxylic acid derivatives: Synthesis, anticancer and antitrypanosomal activity evaluation. Eur. J. Med. Chem. 2014, 75, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Lesyk, R.; Vladzimirska, O.; Holota, S.; Zaprutko, L.; Gzella, A. New 5-substituted thiazolo[3,2-b][1,2,4]triazol-6-ones: Synthesis and anticancer evaluation. Eur. J. Med. Chem. 2007, 42, 641–648. [Google Scholar] [CrossRef] [PubMed]
- Golota, S.; Sydorenko, I.; Surma, R.; Karpenko, O.; Gzella, A.; Lesyk, R. Facile one-pot synthesis of 5-aryl/heterylidene-2-(2-hydroxyethyl- and 3-hydroxypropylamino)-thiazol-4-ones via catalytic aminolysis. Synth. Commun. 2017, 47, 1071–1076. [Google Scholar] [CrossRef]
- Allen, F.H.; Kennard, O.; Watson, D.G.; Brammer, L.; Orpen, A.G.; Taylor, R. Tables of Bond Lengths determined by X-Ray and Neutron Diffraction. Part 1. Bond Lengths in Organic Compounds. J. Chem. Soc. Perkin Trans. 1987, 2, S1–S19. [Google Scholar] [CrossRef]
- Groom, C.R.; Bruno, I.J.; Lightfoot, M.P.; Ward, S.C. The Cambridge Structural Database. Acta Cryst. 2016, B72, 171–179. [Google Scholar] [CrossRef]
- Ambinter. Available online: http://www.ambinter.com (accessed on 28 August 2018).
- Aurora Fine Chemicals. Available online: http://www.aurorafinechemicals.com (accessed on 28 August 2018).
- Zerenex Molecular Ltd. Available online: http://www.zerenex-molecular.com (accessed on 28 August 2018).
- CrysAlis PRO Software system, Version 1.171.38.41; Rigaku Oxford Diffraction, Rigaku Corporation, Oxford, UK: 2015. Available online: https://www.rigaku.com/en/products/smc/crysalis (accessed on 30 September 2018).
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal-structure determination. Acta Cryst. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Cryst. 2015, C71, 3–8. [Google Scholar] [CrossRef]
- Farrugia, L.J. WinGX and ORTEP for windows: An update. J. Appl. Cryst. 2012, 45, 849–854. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds 3 and 4 are available from the authors. |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).