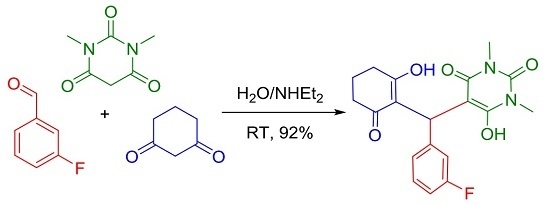
5-[(3-Fluorophenyl)(2-hydroxy-6-oxocyclohex-1-en-1-yl)methyl]-6-hydroxy-1,3-dimethylpyrimidine-2,4(1H,3H)-dione
Abstract
:1. Introduction
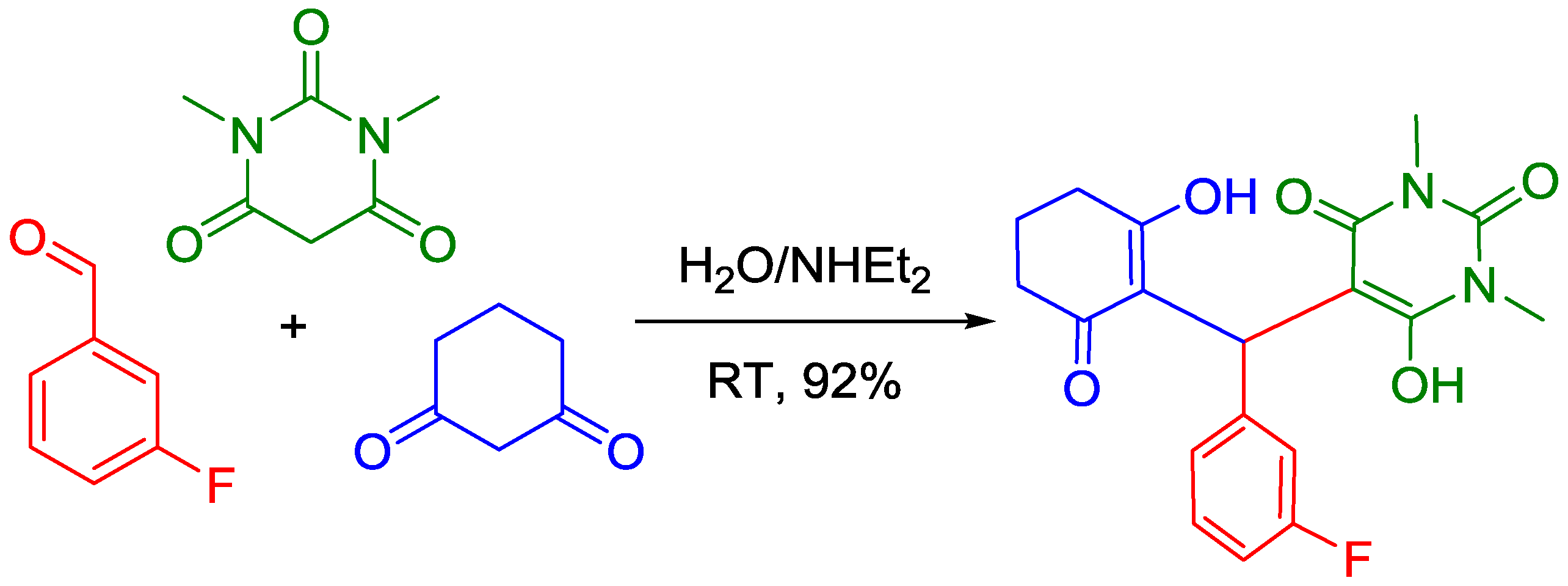
2. Results
3. Discussion
Single-Crystal X-Ray Diffraction Study
4. Materials and Methods
4.1. General
4.2. Synthesis of 5-[(3-Fluorophenyl)(2-hydroxy-6-oxocyclohex-1-en-1-yl)-methyl]-6-hydroxy-1,3-di-methyl-pyrimidine-2,4(1H,3H)-dione
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Bojarski, J.T.; Mokrocz, J.L.; Barton, H.J.; Paluchowska, M.H. Recent progress in barbituric acid chemistry. Adv. Heterocycl. Chem. 1985, 38, 229–297. [Google Scholar]
- Sans, S.R.G.; Chosaz, M.G. Historical aspects and applications of barbituric acid derivatives. Pharmazie 1988, 43, 827–829. [Google Scholar]
- Taylor, J.B. Modern Medical Chemistry; Prentice Hall: New York, NY, USA, 1994. [Google Scholar]
- Holtkamp, M.; Meierkord, H. Anticonvulsant, antiepileptogenic, and antiictogenic pharmacostrategies. Cell. Mol. Life Sci. 2007, 64, 2023–2041. [Google Scholar] [CrossRef] [PubMed]
- Archana, S.; Srivastava, V.K.; Kumar, A. Synthesis of newer indolyl/phenothiazinyl substituted 2-oxo/thiobarbituric acid derivatives as potent anticonvulsant agents. Arzneim. Forsch. Drug Res. 2002, 52, 787–791. [Google Scholar] [CrossRef]
- Barakat, A.; Al-Majid, A.M.; Lotfy, G.; Arshad, F.; Yousuf, S.; Choudhary, M.I.; Ashraf, S.; Ul-Haq, Z. Synthesis and dynamics studies of barbituric acid derivatives as urease inhibitors. Chem. Cent. J. 2015, 9, 63. [Google Scholar] [CrossRef] [PubMed]
- Ul-Haq, Z.; Ashraf, S.; Al-Majid, A.M.; Barakat, A. 3D-QSAR studies on barbituric acid derivatives as urease inhibitors and the effect of charges on the quality of a model. Int. J. Mol. Sci. 2016, 17, 657. [Google Scholar] [CrossRef] [PubMed]
- Goodman, L.S.; Limbird, E.L.; Milinoff, P.B.; Ruddon, R.W.; Gilman, A.G. Goodman and Gilman’s: The Pharmacological Basis of Therapeutics, 9th ed.; McGraw-Hill: New York, NY, USA, 1996; p. 471. [Google Scholar]
- Wolff, M.E. Burger’s Medicinal Chemistry and Drug Discovery; Wiley: New York, NY, USA, 1997. [Google Scholar]
- Chandler, L.J.; Leslie, S.W.; Gonzales, R. 5-(2-Cyclohexylideneethyl)-5-ethyl barbituric acid (CHEB): Correlation of hypnotic and convulsant properties with alterations of synaptosomal 45Ca2+ influx. Eur. J. Pharm. 1986, 126, 117–123. [Google Scholar] [CrossRef]
- Barakat, A.; Al Majid, A.M.A.; Al-Najjar, H.J.; Mabkhot, Y.N.; Javaid, S.; Yousuf, S.; Choudhary, M.I. Zwitterionic pyrimidinium adducts as antioxidants with therapeutic potential for nitric oxide scavenger. Eur. J. Med. Chem. 2014, 84, 146–154. [Google Scholar] [CrossRef] [PubMed]
- Barakat, A.; Soliman, S.M.; Al-Majid, A.M.; Lotfy, G.; Ghabbour, H.A.; Fun, H-K.; Yousuf, S.; Choudhary, M.I.; Wadood, A. Synthesis and structure investigation of novel pyrimidine-2,4,6-trione derivatives of highly potential biological activity as anti-diabetic agent. J. Mol. Strut. 2015, 1098, 365–376. [Google Scholar] [CrossRef]
- Barakat, A.; Islam, M.S.; Al-Majid, A.M.; Ghabbour, H.A.; Fun, H-K.; Javed, K.; Imad, R.; Yousuf, S.; Choudhary, M.I.; Wadood, A. Synthesis, in vitro biological activities and in silico study on dihydropyrimidines derivatives. Bioorg. Med. Chem. 2015, 23, 6740–6748. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.K.; Kumar, P.; Narasimhan, B.; Ramasamy, K.; Mani, V.; Mishra, R.K.; Majeed, A.B.A. Synthesis, antimicrobial, anticancer evaluation and QSAR studies of 6-methyl-4-[1-(2-substituted-phenylamino-acetyl)-1H-indol-3-yl]-2-oxo/thioxo-1,2,3,4-tetrahydropyrimidine-5-carboxylic acid ethyl esters. Eur. J. Med. Chem. 2012, 48, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Al-Majid, A.M.; Barakat, A.; Al-Najjar, H.J.; Mabkhot, Y.N.; Ghabbour, H.A.; Fun, H.-K. Tandem Aldol-Michael reactions in aqueous diethylamine medium: A greener and efficient approach to bis-pyrimidine derivatives. Int. J. Mol. Sci. 2013, 14, 23762–23773. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. 2008, A64, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Brucker. APEX2, SAINT and SADABS, Brucker AXS Inc.: Madison, WI, USA, 2009.
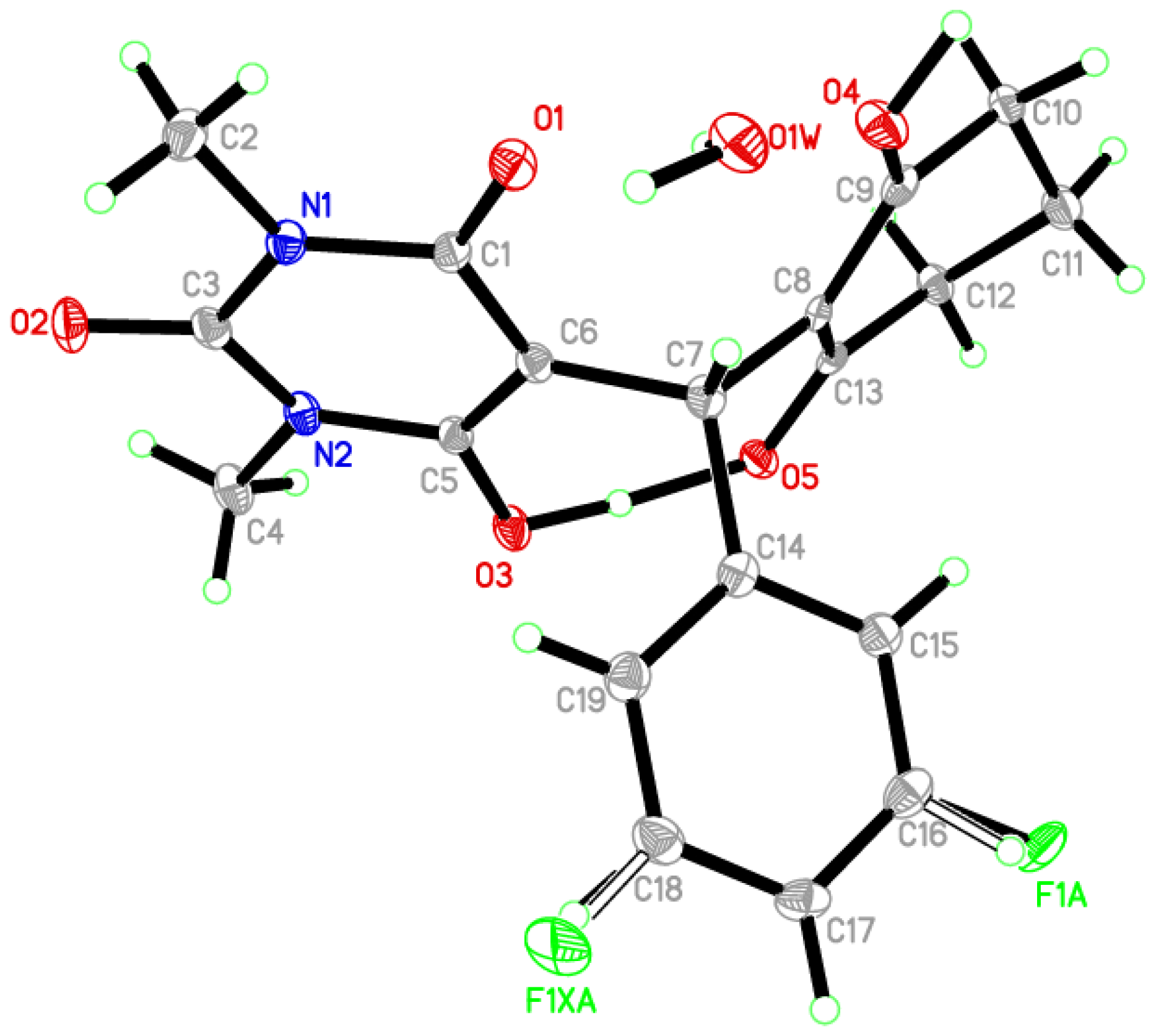

| Crystal Data | |
| Chemical formula | C19H19FN2O5·H2O |
| Mr | 392.38 |
| Crystal system, space group | Monoclinic, P21/c |
| Temperature (K) | 100 K |
| a, b, c (Å) | 7.8630 (5), 20.0308 (13), 11.3987 (8) |
| β (°) | 104.274 (3) |
| V (Å3) | 1739.9 (2) |
| Z | 4 |
| Radiation type | Mo Kα |
| µ (mm−1) | 0.12 |
| Crystal size (mm) | 0.34 × 0.22 × 0.09 |
| Data Collection | |
| Diffractometer | D8 Venture area detector |
| Absorption correction | multi-scan, SADABS V2014/3 |
| No. of measured, independent, and observed [I > 2σ(I)] reflections | 18454, 3070, 1995 |
| (sin θ/λ)max (Å−1) | 0.594 |
| Rint | 0.117 |
| Refinement | |
| R[F2 > 2σ(F2)], wR(F2), S | 0.055, 0.124, 1.05 |
| No. of reflections | 3070 |
| No. of parameters | 288 |
| No. of restraints | 2 |
| H-atom treatment | H atoms treated by a mixture of independent and constrained refinement |
| (Δ/σ)max | 1.208 |
| Δρmax, Δρmin (e Å−3) | 0.28, −0.27 |
| F1–C16 | 1.344 (4) | N1–C1 | 1.411 (4) |
| F1X–C18 | 1.268 (16) | N1–C2 | 1.465 (4) |
| O1–C1 | 1.243 (4) | N1–C3 | 1.370 (4) |
| O2–C3 | 1.229 (4) | N2–C4 | 1.470 (4) |
| O3–C5 | 1.306 (4) | N2–C5 | 1.388 (4) |
| O4–C9 | 1.324 (4) | N2–C3 | 1.374 (4) |
| O5–C13 | 1.257 (4) | ||
| C2–N1–C3 | 116.9 (2) | O3–C5–C6 | 125.3 (3) |
| C1–N1–C3 | 124.3 (3) | O3–C5–N2 | 113.0 (3) |
| C1–N1–C2 | 118.7 (2) | N2–C5–C6 | 121.7 (3) |
| C3–N2–C4 | 118.5 (2) | O4–C9–C10 | 116.9 (3) |
| C3–N2–C5 | 121.9 (3) | O4–C9–C8 | 119.6 (3) |
| C4–N2–C5 | 119.4 (2) | O5–C13–C12 | 116.2 (3) |
| O1–C1–N1 | 117.7 (3) | O5–C13–C8 | 122.7 (3) |
| O1–C1–C6 | 125.3 (3) | F1–C16–C15 | 117.8 (3) |
| N1–C1–C6 | 117.0 (3) | F1–C16–C17 | 118.4 (3) |
| O2–C3–N2 | 121.3 (3) | F1X–C18–C17 | 114.7 (9) |
| N1–C3–N2 | 116.2 (3) | F1X–C18–C19 | 124.3 (9) |
| O2–C3–N1 | 122.5 (3) |
| D–H···A | D–H | H···A | D···A | D–H···A |
|---|---|---|---|---|
| O1W–H2OW···O2 i | 1.09 (6) | 1.74 (6) | 2.815 (3) | 171 (5) |
| O3–H1O3···O5 | 1.02 (4) | 1.44 (4) | 2.454 (3) | 175 (4) |
| O4–H1O4···O1W ii | 1.01 (4) | 1.62 (4) | 2.622 (3) | 173 (4) |
| O1W–H1OW···O1 iii | 1.00 (3) | 1.92 (5) | 2.754 (3) | 138 (4) |
| O1W–H1OW···O4 iii | 1.00 (3) | 2.34 (4) | 3.051 (3) | 127 (4) |
| C2–H2A···F1 iv | 0.9600 | 2.4000 | 3.300 (4) | 156.00 |
| C11–H11B···O3 v | 0.9700 | 2.5400 | 3.288 (4) | 133.00 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barakat, A.; Ghabbour, H.A.; Atef, S.; Al-Majid, A.M.; Shahidul Islam, M.; Ali, M. 5-[(3-Fluorophenyl)(2-hydroxy-6-oxocyclohex-1-en-1-yl)methyl]-6-hydroxy-1,3-dimethylpyrimidine-2,4(1H,3H)-dione. Molbank 2016, 2016, M910. https://doi.org/10.3390/M910
Barakat A, Ghabbour HA, Atef S, Al-Majid AM, Shahidul Islam M, Ali M. 5-[(3-Fluorophenyl)(2-hydroxy-6-oxocyclohex-1-en-1-yl)methyl]-6-hydroxy-1,3-dimethylpyrimidine-2,4(1H,3H)-dione. Molbank. 2016; 2016(4):M910. https://doi.org/10.3390/M910
Chicago/Turabian StyleBarakat, Assem, Hazem A. Ghabbour, Saleh Atef, Abdullah Mohammed Al-Majid, Mohammad Shahidul Islam, and M. Ali. 2016. "5-[(3-Fluorophenyl)(2-hydroxy-6-oxocyclohex-1-en-1-yl)methyl]-6-hydroxy-1,3-dimethylpyrimidine-2,4(1H,3H)-dione" Molbank 2016, no. 4: M910. https://doi.org/10.3390/M910
APA StyleBarakat, A., Ghabbour, H. A., Atef, S., Al-Majid, A. M., Shahidul Islam, M., & Ali, M. (2016). 5-[(3-Fluorophenyl)(2-hydroxy-6-oxocyclohex-1-en-1-yl)methyl]-6-hydroxy-1,3-dimethylpyrimidine-2,4(1H,3H)-dione. Molbank, 2016(4), M910. https://doi.org/10.3390/M910