Abstract
5,9,11-Trihydroxy-2,2-dimethyl-10-(3′-methyl-2′-butenyl)-3-(2″-methyl-3″-butenyl)-pyrano[2,3-a]xanthen-12(2H)-one (1) was isolated from the stem bark of Calophyllum pseudomole. The structure of 1 was established by spectroscopic analysis which included UV, IR, HRESIMS and NMR experiments.
1. Introduction
The Calophyllum genus (Clusiaceae) comprises more than 180 species found mainly in Southeast Asia. This genus has been shown to produce a number of secondary metabolites, particularly xanthones [1,2,3], coumarins [4,5,6], chromanone acids [7,8,9], and flavonoids [10]. In Indonesia, the local name of Calophyllum is ‘bitangor’ [11].
Herein, we report the isolation and structural elucidation of a new isoprenylated xanthone, 5,9,11-trihydroxy-2,2-dimethyl-10-(3′-methyl-2′-butenyl)-3-(2″-methyl-3″-butenyl)-pyrano[2,3-a]xanthen-12(2H)-one (1) (Figure 1) from the stem bark of Calophyllum pseudomole as well as its antioxidant activity.
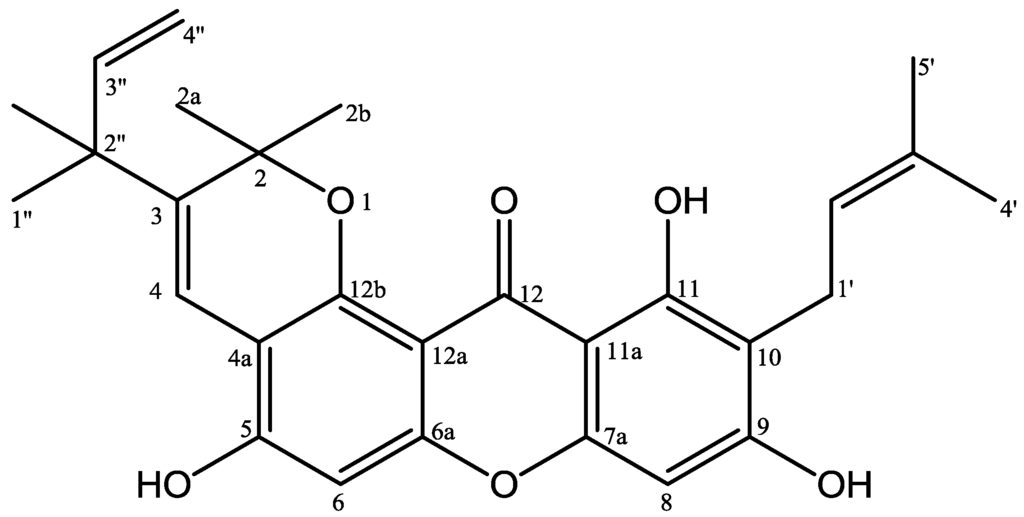
Figure 1.
Structures of 5,9,11-trihydroxy-2,2-dimethyl-10-(3′-methyl-2′-butenyl)-3-(2″-methyl-3″-butenyl)pyrano[2,3-a]xanthen-12(2H)-one (1).
2. Result and Discussion
5,9,11-Trihydroxy-2,2-dimethyl-10-(3′-methyl-2′-butenyl)-3-(2″-methyl-3″-butenyl)pyrano[2,3-a]xanthen-12(2H)-one (1) was isolated as a yellow solid, m.p. 160–162 °C. The molecular formula of compound is C28H30O6, whereas that of the deprotonated molecule [M − H]− is C28H29O6 at m/z 461.1971 (calcd. 461.1964) by the HRESIMS. The UV spectrum exhibited four absorption bands characteristic of a xanthone chromophore at λmaks 247, 264, 322 and 396 nm [1]. The IR spectrum showed absorption bands at νmax 3423, 1622, and 1460 cm−1 indicating the presence of a hydroxyl, conjugated carbonyl and aromatic groups, respectively. The 1H-NMR (Table 1) spectrum showed the presence of a chelated hydroxyl group (δH 13.77, 11-OH) and two isolated aromatic proton signals at δH 6.77 (1H, s, H-6) and 6.40 (1H, s, H-8) suggest that compound 1 is similar to a xanthone with six substituents [1]. The 1H-NMR also revealed signals due to 3′-methyl-2′-butenyl group [δH 1.63 (3H, s, H-4′), 1.77 (3H, s, H-5′), 3.34 (2H, d, J = 7.3 Hz, H-1′), 5.27 (1H, t, J = 7.3 Hz, H-2′)], ring of 2,2-dimethylpyrano monosubstituent group at δH 1.49 (6H, s, H2a/H-2b), 8.19 (1H, s, H-4) and 1,1-dimethylalyl group [δH 1.41 (6H, s, H-1″, 2″-CH3), 5.08 (1H, dd, J = 1.1; 10.6 Hz, H-4″a), 5.16 (1H, dd, J = 1.1; 17.5 Hz, H-4″b), 6.02 (1H, dd, J = 10.6; 17.5 Hz, H-3″)]. The 13C-NMR spectrum (Table 1) of 1, 26 carbon signals representing 28 carbon atoms were observed. The HMBC spectrum, the chelated hydroxyl group (δH 13.77, 11-OH) correlated with three quaternary carbons [δC 161.5 (C-11), 110.9 (C-10), 103.8 (C-11a)], and two carbons being further correlated to the isolated aromatic (δH 6.40), indicating that the para-position of the hydroxyl group was unsubstituted. The presence of long-range correlations in the HMBC spectrum between methylen group at δH 3.34 on the isoprenyl group with three aromatic carbon signals at δC 162.9 (C-9), 161.5 (C-11), 110.9 (C-10) and two vinyl carbon signals at δC 131.4 (C-3′), 118.8 (C-2′), indicated that an isoprenyl is attached at C-10 proton. Furthermore, a proton signal of an aromatic (δH 6.77, H-6) correlated with three quaternary carbons [δC 154.1 (C-5), 153.3 (C-6a), 108.4 (C-4a)] showed 2,2-dimethylpyrano group were fused at C-4a and C-12b. The presence of long-range correlations between vinyl group at δH 8.19 the that 2,2-dimethylpyrano group with four quaternary carbons [δC 137.6 (C-3), 108.4 (C-4a), 80.3 (C-2), 42.7 (C-2″)] showed that 1,1-dimethylalyl group attached at C-3. Therefore, compound 1, was elucidated as 5,9,11-trihydroxy-2,2-dimethyl-10-(3′-methyl-2′-butenyl)-3-(2″-methyl-3″-butenyl)pyrano[2,3-a]xanthen-12(2H)-one. Other HMBC correlations consistent with the structure 1 are shown in Table 1 and Figure 2. To our knowledge, compound 1 has not been reported previously as a novel natural product.

Table 1.
NMR spectroscopic data of 5,9,11-trihydroxy-2,2-dimethyl-10-(3′-methyl-2′-butenyl)-3-(2″-methyl-3″-butenyl)pyrano[2,3-a]xanthen-12(2H)-one in acetone-d6.
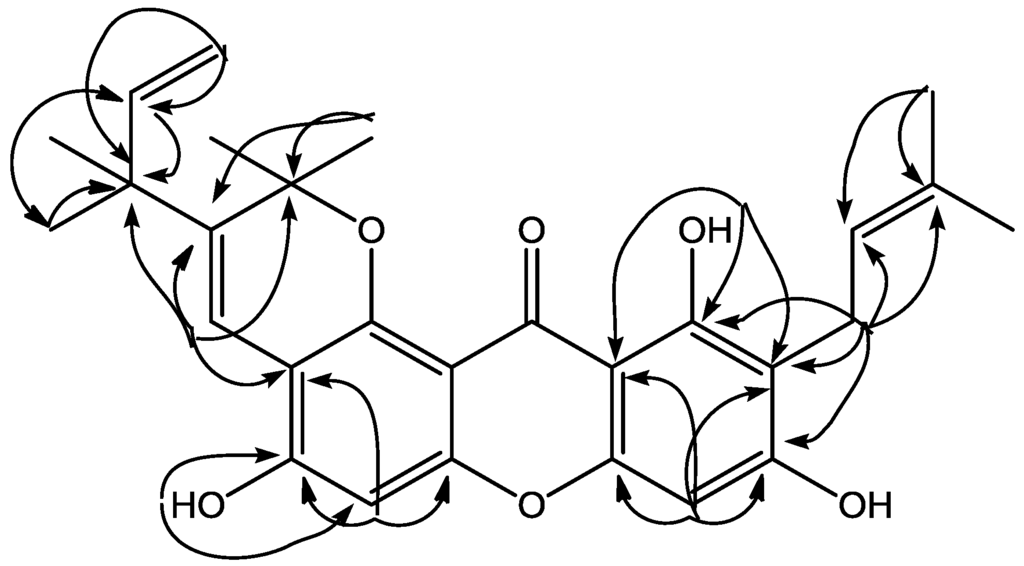
Figure 2.
Selected HMBC correlations for 1.
On antioxidant evaluation against DPPH radical scavenging, compound 1 exhibited IC50 values 76 μg/mL more active than apigenin as control positive (IC50 130 μg/mL). Those antioxidant data suggested that compound 1 has high activity.
3. Experimental Section
3.1. General
The UV spectrum was measured with Shimadzu series 1800 spectrophotometer (Kyoto, Japan). The IR spectrum was recorded with Perkin-Elmer spectrum-100 FT-IR (Waltham, MA, USA). NMR spectra were recorded on a JEOL 400 ECA spectrophotometer (Tokyo, Japan) in acetone-d6 at 400 (1H) and 100 (13C) MHz using TMS as the internal standard. The mass spectra were recorded using a Waters LCT Premier XE (Santa Clara, CA, USA). Column chromatography and radial chromatography were carried out using silica gel 60 and silica gel 60 PF254 (Merck, Darmstadt, Germany).
3.2. Plant Material
The stem bark of C. pseudomole was collected in Sungai Mendawak, anak Sungai Kapuas, District Kubu Raya, Kalimantan, Indonesia on April 2015. The sample was identified and deposited in the Herbarium Bogoriense, Center of Biological Research and Development, National Institute of Science, Bogor, Indonesia.
3.3. Extraction and Isolation
The dried stem bark of C. pseudomole (3.0 kg) was macerated in methanol twice for 4 days, and then evaporated under reduced pressure to give a dark brown residue (120 g). Further, the methanol extract was partitioned first with n-hexane. The methanol extract was mixed with water (10% v/v) to increase the polarity and then partitioned with ethyl acetate. The ethyl acetate extract (24 g) was subjected to column chromatography over silica gel and eluted with n-hexane-ethyl acetate (from 9:1 to 3:7) to give fractions A–D. Fraction B showed the most potent antioxidant activity. Fraction B was then subjected to column chromatography and eluted with n-hexane-ethyl acetate (from 9:1 to 7:3) to produce subfractions B1–B3. Subfraction B2 was purified by planar radial chromatography using n-hexane-acetone (from 9:2 to 4:1) to yield compound 1 (16 mg).
3.4. DPPH Radical Scavenging
The antioxidant assay of compound 1 against DPPH (2,2-diphenyl-1-picrihidrazil) radical was measured by UV spectrometer at λ 517 nm as described previously [12,13,14]. The inhibition percentage (%) of radical scavenging activity was calculated using the following equation:
where Ao is the absorbance of the control reaction (containing all reagents except the active compound), and As is the absorbance of the active compound.
Inhibition (%) = (Ao − As/Ao) × 100
Supplementary Materials
HRESIMS, 1H-NMR, 13C-NMR, HMQC, HMBC, IR and UV spectra are reported in the supplementary materials at www.mdpi.com/1422-8599/2016/3/M906.
Supplementary File 1Acknowledgments
This research was supported by Directorate General of Strengthening Research and Development, Ministry of Research, Technology and Higher Education, Republic of Indonesia (Penelitian Hibah Kompetensi, Universitas Airlangga, 2016).
Author Contributions
Tjitjik Sri Tjahjandarie designed the whole experiment of bioactivity and contributed to the manuscript. Mulyadi Tanjung researched data, analyzed the NMR and HRESIMS spectra and wrote the manuscript, Ratih Dewi Saputri designed the whole experiment. All authors read and approved the final manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ito, C.; Itoigawa, M.; Mishina, Y.; Filho, V.C.; Mukainaka, T.; Tokuda, H.; Nishino, H.; Furukawa, H. Chemical constituents of Calophyllum brasiliensis: Structure elucidation of seven xanthones and their cancer chemopreventive activity. J. Nat. Prod. 2002, 65, 267–272. [Google Scholar] [CrossRef] [PubMed]
- Morel, C.; Seraphin, D.; Oger, J.M.; Litaudon, M.; Sevenet, T.; Richomme, P.; Bruneton, J. New xanthones from Calophyllum caledonicum. J. Nat. Prod. 2000, 63, 1471–1474. [Google Scholar] [CrossRef] [PubMed]
- Wei, D.J.; Mei, W.L.; Zhong, H.M.; Zeng, Y.B.; Wu, X.D.; Dai, H.F. A new prenylated xanthone from the branches of Calophyllum inophyllum. J. Asian Nat. Prod. Res. 2011, 13, 265–269. [Google Scholar] [CrossRef] [PubMed]
- Joshi, S.P.; Kulkarni, S.R.; Phalgune, U.D.; Puranik, V.G. New dipyranocoumarin from the leaves of Calophyllum apetalum Willd. Nat. Prod. Res. 2013, 27, 1896–1901. [Google Scholar] [CrossRef] [PubMed]
- Guilet, D.G.; Helesbeux, J.J.; Seraphin, D.; Sevenet, T.; Richomme, P.; Bruneton, J. Novel cytotoxic 4-phenilcoumarins from Calophyllum dispar. J. Nat. Prod. 2001, 64, 563–568. [Google Scholar] [CrossRef] [PubMed]
- Daud, S.B.; Ee, G.C.L.; Malek, E.A.; Teh, S.S.; See, I. A new coumarin from Calophyllum hosei. Nat. Prod. Res. 2014, 28, 1534–1538. [Google Scholar] [CrossRef] [PubMed]
- Reyes, M.H.; Basualdo, M.C.; Abe, F.; Estrada, M.J.; Soler, C.; Chilpa, R.R. HIV-1 inhibitory compounds from Calophyllum brasiliense leaves. Biol. Pharm. Bull. 2004, 27, 1471–1475. [Google Scholar] [CrossRef]
- Ha, L.D.; Hansen, P.E.; Duus, F.; Pham, H.D.; Nguyen, L.D. A new chromanone acid from the bark of Calophyllum dryobalanoides. Phytochem. Lett. 2012, 5, 287–291. [Google Scholar] [CrossRef]
- Cottiglia, F.; Dhanapal, B.; Sticher, O.; Heilmann, J. New chromanone acids with antibacterial activity from the bark of Calophyllum brasiliense. J. Nat. Prod. 2004, 67, 537–541. [Google Scholar] [CrossRef] [PubMed]
- Ferchichi, L.; Derbre, S.; Mahmood, K.; Toure, K.; Guilet, D.; Litaudon, M.; Awang, K.; Hadi, A.H.A.; Ray, A.M.L.; Richomme, P. Bioguided fractionation and isolation of natural inhibitors of advanced glycation end-products (AGEs) from Calophyllum flavoramulum. Phytochemistry 2012, 78, 98–106. [Google Scholar] [CrossRef] [PubMed]
- Burkill, I.H. A Dictionary of the Economic Products of the Malay Peninsula; Goverment of Malaysia and Singapore by the Ministry of Agriculture and Co-Operatives: Kuala Lumpur, Malaysia, 1966; pp. 410–417.
- Tjahjandarie, T.S.; Saputri, R.D.; Tanjung, M. Methyl 2,5-dihydroxy-4-(3′-methyl-2′-butenyl)benzoate. Molbank 2016, M892. [Google Scholar] [CrossRef]
- Tanjung, M.; Tjahjandarie, T.S.; Sentosa, M.H. Antioxidant and cytotoxic agent from the rhizomes of Kaempferia pandurata. Asian Pac. J. Trop. Dis. 2013, 3, 401–404. [Google Scholar] [CrossRef]
- Tanjung, M.; Saputri, R.D.; Tjahjandarie, T.S. Antioxidant activity of two isomeric benzoxepin derivatives from the stem bark of Bauhinia acuelata L. J. Chem. Pharm. Res. 2014, 6, 705–708. [Google Scholar]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).