Abstract
Environmentally friendly and straightforward methods for creating biofuels are required to promote biofuel use. Therefore, we present here a convenient and environmentally friendly direct self-aldol reaction of acetaldehyde in 100 mM borate buffer (pH 10) affording the dimer of 3-hydroxybutanal with a good yield. The product can be easily converted into 1,3-butanediol and its benzoate; therefore, our results will have a positive impact in the field of biochemical production from ethanol.
1. Introduction
A biorefinery is a system in which renewable biomass substances are converted to bio-based products. The system is now the focus of worldwide attention as an alternative to the oil refinery system. High and volatile oil prices, coupled with increasing CO2 concentrations in the atmosphere causing the greenhouse effect, has been an important social catalyst in organic chemistry to produce bio-based products using biomass as the starting material with environmentally friendly reactions [1,2,3]. In developed countries, most governments are prioritizing research on bioethanol as well as the development of a bioethanol industry, in particular for fuel purposes. Previous studies on practical bioethanol production had shown that the cost of producing bioethanol was surprisingly high and that reducing this cost was key to making bioethanol economically viable. Subsequently, a lot of effort was expended on increasing the cost effectiveness of producing bioethanol, and the cost of bioethanol has been dramatically reduced [4,5,6].
We therefore tried using bioethanol as a starting material in an environmentally friendly synthesis reaction to obtain 1,3-butanediol (2), which is used in large quantities and is a very important industrial raw material [7,8]. 1,3-Butanediol (2) can be synthesized from ethanol using the following steps: Step (1) an ethanol molecule is oxidized to an acetaldehyde; Step (2) the acetaldehyde molecule provides 3-hydroxybutanal via a direct self-aldol reaction; and Step (3) 1,3-butanediol (2) is obtained by reduction of 3-hydroxybutanal. However, the direct self-aldol reaction of acetaldehyde in Step 2 is difficult because the generated aldehyde acts both as a reactive electrophile and as a nucleophile, causing overreactions. In this paper, we describe the first successful realization of the direct self-aldol reaction of acetaldehyde under environmentally friendly reaction conditions, i.e., catalyst free and using water as the reaction media.
2. Results and Discussion
In the direct self-aldol reaction of acetaldehyde, the high reactivity of 3-hydroxybutanal, which can act as both an electrophile and a nucleophile, becomes a problem [9,10,11,12,13]. To solve this problem, the hydroxyl and aldehyde groups of 3-hydroxybutanal are reacted and converted into an acetal. In other words, an acetal dimerization of 3-hydroxybutanal is carried out to simultaneously mask the reactivity of the hydroxyl and aldehyde groups. Furthermore, this should be achieved using environmentally friendly reaction conditions, for example, using water as the reaction solvent. To achieve a direct self-aldol reaction of acetaldehyde and immediate subsequent dimerization of the aldol product, a two-step reaction from acetaldehyde was examined, focusing on the variety of combinations of conditions such as the pH of reaction media, acetaldehyde concentration, reaction time, and reaction temperature.
The results are summarized in Table 1 and Table 2. The best result of the two-step reaction (direct self-aldol reaction of acetaldehyde and dimerization reaction) in water was obtained when 5 M acetaldehyde in 100 mM Na borate buffer, pH 10, and a 48 h reaction at ambient temperature were employed to afford compound 1 with a 69% yield (Table 1, Entry 4 and Table 2, Entry 7).

Table 1.
Optimizing the direct self-aldol reaction and dimerization reaction conditions under pH 7 to 10 using 5 M acetaldehyde.

Table 2.
Optimizing the direct self-aldol reaction and dimerization reaction conditions under different acetaldehyde concentrations and reaction time at pH 10.
We were able to isolate compound 1 with good yield, as compound 1 is relatively stable on silica gel. The NMR and MS of compound 1 suggested that dimer was the main product. Compound 1 was reduced to 1,3-butanediol using NaBH4 in MeOH, and then the product was benzoylated. The isolated yield of benzoylated product 3 synthesized in two steps was determined to be 81%. The reaction scheme is summarized in Scheme 1.
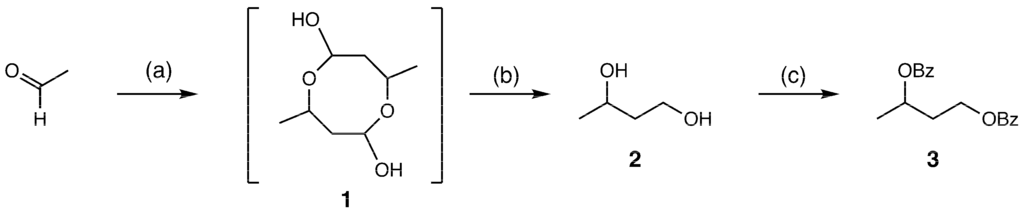
Scheme 1.
Synthesis of 1,3-butanediol dibenzoate (3). Reagents and conditions: (a) 100 mM borate buffer pH 10, ambient temperature, 2 days, with yield of 69%; (b) NaBH4, MeOH, 0 °C, 1 h; (c) PhCOCl, Et3N, DMAP, CH2Cl2, ambient temperature, 18 h, with yield of 81% (2 steps).
We conducted a high-performance liquid chromatography (HPLC) analysis of the compounds 3R and 3S. The compound 2R was benzoylated according to the conventional method (Scheme 2). Retention times of 3R and 3S were determined by the HPLC condition using synthesized racemic 3 and 3R. The retention times of 3R and 3S were 14.8 min and 22.0 min, respectively. In the future, it is important to develop asymmetric and catalytic versions of the direct self-aldol reaction of acetaldehyde in water media [10].
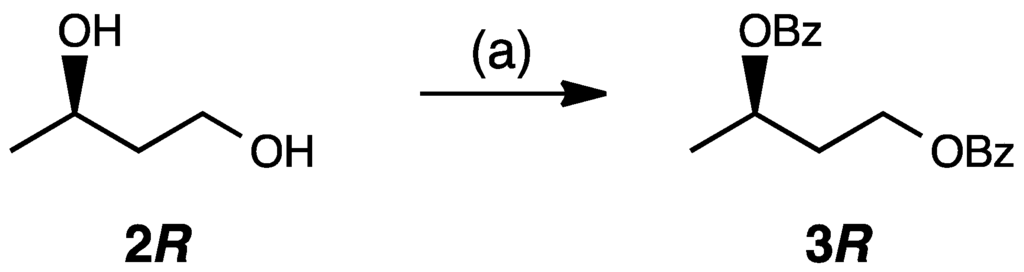
Scheme 2.
Synthesis of (R)-1,3-butanediol dibenzoate (3R). Reagents and conditions: (a) PhCOCl, Et3N, DMAP, CH2Cl2, 18 h, ambient temperature, with yield of 83%.
Sustainable production of chemicals remains essential both commercially and scientifically. 1,3-Butanediol has gained significant attention as an important commodity chemical with broad applications. Bioethanol can be converted to 1,3-butanediol without the use of a catalyst in water through a self-aldol reaction of acetaldehyde. The experimental results demonstrate that the acetaldehyde concentrations and pH of water were particularly important for the desired self-aldol reaction. All these results indicate the promising application of the developed self-aldol reaction of acetaldehyde in producing useful biochemicals from bioethanol.
3. Experimental Section
3.1. General Information
The 1H spectra of the compounds were recorded on an ECA500 spectrometer (JEOL, Tokyo, Japan) after the compounds were dissolved in CDCl3; Tetramethylsilane was used as an internal standard. The coupling constants (J) are reported in Hz and refer to the apparent peak multiplicities (s = singlet, d = doublet, dd = double doublet, ddd = triple doublet, m = multiple). Low-resolution mass spectra were obtained with a Quattro Premier XE instrument (Waters, Milford, MA, USA) under positive and negative ion electrospray ionization conditions. Column chromatography was performed using Silica Gel 60N with a spherical neutral particle size of 100–210 µm (Kanto Chemical, Tokyo, Japan). The progress of all reactions was monitored by thin-layer chromatography on Silica Gel 60 F254 0.25 mm (E. Merck, Darmstadt, Germany). Optical rotations were measured with a P-1020 polarimeter (JASCO, Tokyo, Japan) at 25 °C. HPLC was performed on a Hitachi LaChrom Elite system by Hitachi High Technologies Inc., Tokyo, Japan (UV detector: L-2400; pump: L-2130; column oven: L-2300 (30 °C); column: CHIRALCEL OD-H (4.6 mm × 250 mm, DAICEL, Tokyo, Japan); isocratic eluent: hexane/2-propanol 93:7; flow rate = 1.0 mL/min; wavelength: 240 nm; and overall run time: 40 min).
3.2. Synthesis of 1,3-Butanediol Dibenzoate (3) from Acetaldehyde
To make 25 mL of 5 M acetaldehyde solution, 5.5 g of freshly prepared acetaldehyde Sigma-Aldrich (St. Louis, MI, USA, 00070-100ML) was dissolved in 100 mM sodium borate buffer (pH 10), fill up to 25 mL. The 25 mL of 5 M acetaldehyde solution was stirred at ambient temperature for 2 days. The reaction mixture was poured into ethyl acetate and the product was extracted three times with ethyl acetate. The organic layer was dried over Na2SO4 and then evaporated. The obtained residue was purified by column chromatography on silica gel (1:1, hexane:ethyl acetate) to afford 3.78 g (69% yield) of 3-hydroxybutanal dimer (1).
To a stirred solution of freshly purified 3-hydroxybutanal dimer (203 mg, 1.15 mmol) in MeOH (5 mL), NaBH4 (143.3 mg, 3.11 mmol, assay 90%) was added at 0 °C. The stirring was continued at this temperature for 1 h. Afterwards, the reaction mixture was directly processed using column chromatography on silica gel (25:1, diethylether:hexane) to furnish the reduced product, which consisted mainly of compound 2. This product was diluted with dry CH2Cl2 (10 mL) and Et3N (860 µL, 6.18 mmol), DMAP (113.7 mg, 0.93 mmol), and PhCOCl (540 µL, 4.64 mmol) were added to it at 4 °C, and the solution was stirred for 18 h. Subsequently, the reaction mixture was quenched with 1 M HCl (50 mL), extracted with ethyl acetate (50 mL), and washed with saturated NaHCO3 and brine. The organic layer was dried over Na2SO4 and then evaporated. The residue obtained was purified by column chromatography on silica gel (5:1, hexane:ethyl acetate) to afford 557.5 mg (1.87 mmol, 81% Yield) of 1,3-butanediol dibenzoate (3), see Figures S2 and S4 for more information.
1H-NMR (500 MHz, CDCl3): δ 1.45 (d, 3H, J = 6.5 Hz), 2.12–2.26 (m, 2H), 4.42 (ddd, 1H, J = 4.9 Hz, J = 6.6 Hz, J = 13 Hz), 4.50 (dd, 1H, J = 5.8 Hz, J = 11.5 Hz), 5.40 (m, 1H), and 7.41–8.04 (m, 10H). ESI-MS (Positive mode); m/z = 321 [M + Na]+. Spectroscopic data for characterization can be found in reference [10].
3.3. Synthesis of (R)-1,3-Butanediol Dibenzoate (3R)
To (R)-1,3-butanediol (2R, 100 mg, 1.11 mmol, Tokyo Chemical Industry, Tokyo, Japan) in dry CH2Cl2 (10 mL), Et3N (619 µL, 4.44 mmol), DMAP (81.4 mg, 0.66 mmol), and PhCOCl (387 µL, 3.33 mmol) were added at 4 °C, and the solution was stirred for 18 h. After that, the reaction mixture was quenched with 1 M HCl (50 mL), extracted with ethyl acetate (50 mL), and washed with saturated NaHCO3 and brine. The organic layer was dried over Na2SO4 and then evaporated. The residue obtained was purified by column chromatography on silica gel (5:1, hexane:ethyl acetate) to afford 293.3 mg (0.75 mmol, 83% yield) of (R)-1,3-butanediol dibenzoate (3R), see Figures S1 and S3 for more information.
1H-NMR (500 MHz, CDCl3): δ 1.45 (d, 3H, J = 6.5 Hz), 2.12–2.26 (m, 2H), 4.42 (ddd, 1H, J = 4.6 Hz, J = 6.6 Hz, J = 13.3 Hz), 4.50 (dd, 1H, J = 5.6 Hz, J = 11.3 Hz), 5.40 (m, 1H), and 7.41–8.04 (m, 10H). ESI-MS (Positive mode); m/z = 321 [M + Na]+ and [α]D = −68.9 (c 1.0, CHCl3). Spectroscopic data for characterization can be found in reference [10].
Supplementary Materials
The following are available online at http://www.mdpi.com/1422-8599/2016/3/M905.
Supplementary File 1Supplementary File 2Supplementary File 3Supplementary File 4Supplementary File 5Supplementary File 6Supplementary File 7Acknowledgments
This research was supported in part by a grant from the College of Bioresource Sciences, Nihon University.
Author Contributions
S.I., R.O., and R.S. optimized the synthesis reaction of compound 1. K.G. optimized the reaction, acquired the spectra, and analyzed the data of compounds 3 and 3R. A.I. and S.Y. performed the HPLC analysis of compounds 3R and 3S. W.H., T.H. and T.N. designed the synthesis reactions, confirmed the data analysis, and wrote the paper. All of the authors read and approved the final manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Scarlat, N.; Dallemand, J.F.; Monforti-Ferrario, F.; Nita, V. The role of biomass and bioenergy in future bioeconomy: Policies and facts. Environ. Dev. 2015, 15, 3–34. [Google Scholar] [CrossRef]
- Cheali, P.; Quaglia, A.; Gernaey, K.V.; Sin, G. Effect of market price uncertainties on the design of optimal biorefinery systems—A systematic approach. Ind. Eng. Chem. Res. 2014, 53, 6021–6032. [Google Scholar] [CrossRef]
- Bozell, J.J.; Petersen, G.R. Cutting-edge research for a greener sustainable future Technology development for the production of biobased products from biorefinery carbohydrates—The US Department of Energy’s “Top 10” revisited. Green Chem. 2010, 12, 539–554. [Google Scholar] [CrossRef]
- Long, X.; Ji, X. A review of the ecological and socioeconomic effects of biofuel and energy policy recommendations. Renew. Sustain. Energy Rev. 2016, 61, 41–52. [Google Scholar]
- Wang, K.; Ou, L.; Brown, T.; Brown, R.C. Beyond ethanol: A techno-economic analysis of an integrated corn biorefinery for the production of hydrocarbon fuels and chemicals. Biofuels Bioprod. Biorefin. 2014, 16, 177–189. [Google Scholar] [CrossRef]
- George, A.; Brandt, A.; Tran, K.; Zahavi, S.M.N.S.; Klein-Marcuschamer, D.; Sun, N.; Sathitsuksanoh, N.; Shi, J.; Stavila, V.; Parthasarathi, R.; et al. Design of low-cost ionic liquids for lignocellulosic biomass pretreatment. Green Chem. 2015, 17, 1728–1734. [Google Scholar] [CrossRef]
- Kataoka, N.; Vangnai, A.S.; Ueda, H.; Tajima, T.; Nakashimada, Y.; Kato, J. Enhancement of (R)-1, 3-butanediol production by engineered Escherichia coli using a bioreactor system with strict regulation of overall oxygen transfer coefficient and pH. Biosci. Biotechnol. Biochem. 2014, 78, 695–700. [Google Scholar] [CrossRef] [PubMed]
- Matsuyama, A.; Yamamoto, H.; Kawada, N.; Kobayashi, Y. Industrial production of (R)-1,3-butanediol by new biocatalysts. J. Mol. Catal. B Enzym. 2001, 11, 513–521. [Google Scholar] [CrossRef]
- Liu, Q.; Xu, G.; Wang, X.; Mu, X. Selective upgrading of ethanol with methanol in water for the production of improved biofuel—Isobutanol. Green Chem. 2016, 18, 2811–2818. [Google Scholar] [CrossRef]
- Kim, S.M.; Kim, Y.S.; Kim, D.W.; Rios, R.; Yang, J.W. Acetaldehyde: A small organic molecule with big impact on organocatalytic reactions. Chem. Eur. J. 2016, 22, 2214–2234. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Kumar, A.; Rizvi, M.A.; Shah, B.A. Acetaldehyde in asymmetric organocatalytic transformations. RSC Adv. 2015, 5, 55926–55937. [Google Scholar] [CrossRef]
- Hayashi, Y.; Samanta, S.; Itoh, T.; Ishikawa, H. Asymmetric, catalytic, and direct self-aldol reaction of acetaldehyde catalyzed by diarylprolinol. Org. Lett. 2008, 10, 5581–5583. [Google Scholar] [CrossRef] [PubMed]
- Córdova, A.; Notz, W.; Barbas, C.F., III. Proline-catalyzed one-step asymmetric synthesis of 5-hydroxy-(2E)-hexenal from acetaldehyde. J. Org. Chem. 2002, 67, 301–303. [Google Scholar] [CrossRef] [PubMed]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).