Abstract
Chitooligosaccharide with one 2,5-anhydro-d-mannofuranose unit at the reducing end (COSamf) was prepared by nitrous acid depolymerization of chitosan. The reducing-end functionalization of COSamf by reductive amination with octanoic hydrazide in the presence of NaBH3CN was achieved in high yield. The chemical structure of the targeted octanoic hydrazide-linked COSamf was fully characterized by NMR spectroscopy and MALDI-TOF mass spectrometry. This synthesis opens the way to a new generation of COSamf derivatives with potential amphiphilic properties.
1. Introduction
Chitosan is a linear copolymer of β-(1→4)-linked d-glucosamine (GlcN) and N-acetyl d-glucosamine (GlcNAc) units in various proportions. Although present to a low extent in biomass, chitosan is generally obtained by chemical or enzymatic N-deacetylation of chitin, the second most abundant naturally occurring polymer produced industrially from shells of crustaceans and squid pens [1,2,3,4]. Chitin and chitosan oligomers, also named chitooligosaccharides (COS), have recently received considerable attention as functional biomolecules with a wide range of potential applications in food, agriculture, medicine, pharmaceutics and cosmetics [5,6]. COS take advantage of their various interesting physico-chemical and biological properties, including principally water-solubility, biocompatibility, and antibacterial, antiviral and antifungal activities [7,8,9,10]. In order to develop new high-potential COS-based materials with advanced and significant value-added applications, the chemical modification of COS is currently being explored intensively [11,12,13,14,15]. In this study, we described the original synthesis of octanoic hydrazide–linked chitooligosaccharide-2,5-anhydro-d-mannofuranose. The interest of this work is to take advantage of the reactivity of the “non-masked” aldehyde group of the 2,5-anhydro-d-mannofuranose (amf) unit present at the reducing end of COS produced by the nitrous acid depolymerization of chitosan. It should open opportunities for preparing numerous COS-based functional materials with amphiphilic properties.
2. Results and Discussion
Octanoic hydrazide–linked chitooligosaccharide-2,5-anhydro-d-mannofuranose was efficiently synthesized from chitosan in a two-step procedure involving the reductive amination of chitooligosaccharide-2,5-anhydro-d-mannofuranose (COSamf 1) with octanoic hydrazide as illustrated in Scheme 1.
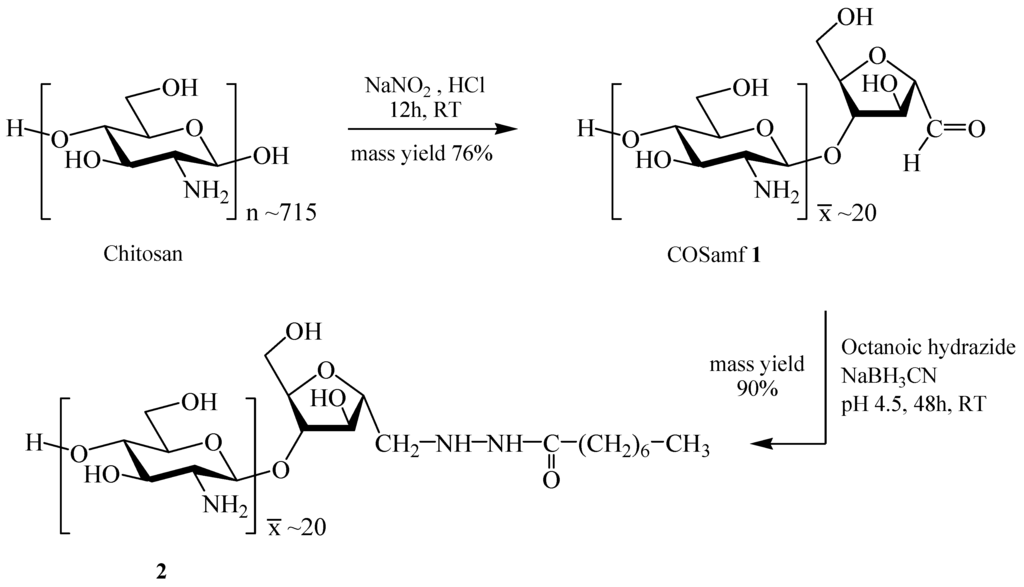
Scheme 1.
Synthesis of octanoic acid–linked chitooligosaccharide-2,5-anhydro-d-mannofuranose (2) from chitosan.
COSamf 1 was prepared by nitrous acid depolymerization of fully N-deacetylated chitosan based on the method previously described by Tommeraas et al. [16]. Thus, the depolymerization of chitosan by sodium nitrite (NaNO2; GlcN/NaNO2 molar ratio = 10:1) in aqueous acid solution at room temperature led to COSamf 1 in 76% mass yield after 12 h of reaction. The chemical structure of COSamf 1 was fully confirmed by NMR (1H and 13C) spectroscopy and MALDI-TOF mass spectrometry. It has been shown by MALDI-TOF analyses that COSamf 1 is composed of a mixture of oligomers with an average number of GlcN units () into chains around 20. The value was determined by 1H-NMR from the relative peak intensities of H-4 (amf) and H-2 (GlcN) signals at 4.23 and 3.15 ppm, respectively, according to the formula (1):
The reductive amination of COSamf 1 with octanoic hydrazide in the presence of NaBH3CN was carried out at room temperature in ethanol/acetate buffer solution (pH 4.5) for 48 h, leading to the targeted octanoic hydrazide–linked COSamf 2 in excellent mass yield (90%) after purification. The chemical structure of compound 2 was entirely characterized by 1H- and 13C-NMR spectroscopies thanks to two-dimensional NMR analyses (COSY, HSQC and HMBC), pointing out the coupling reaction between the aldehyde function of COSamf 1 and the amine group of the hydrazide residue. Thus, the presence of the corresponding CH2-N covalent linkage was displayed at δ 3.37 ppm for the methylene protons and at 52.3 ppm for the methylene carbon in 1H- and 13C-NMR spectra, respectively. As confirmed by MALDI-TOF mass spectrometry (see supplementary materials for more details), octanoic hydrazide–linked COSamf 2 is composed of a mixture of oligomers with a value determined by 1H-NMR equal to around 20. This result confirms that reductive amination conditions used in this study did not affect the GlcN backbone of the starting COSamf 1 .
3. Materials and Methods
3.1. Materials and Analytical Methods
Commercial chitosan (batch 244/020208; degree of N-acetylation: DA < 1%, = 270 kg/mol; = 115 kg/mol, dispersity: Ð = 2.3) was supplied by Mahtani Chitosan Ltd. (Veraval, India). Sodium nitrite (assay > 99%), octanoic hydrazide (assay > 80%), deuterium oxide (D2O, assay > 99.96% atom D), all others chemicals and solvents were provided by Sigma-Aldrich (Saint-Quentin Fallavier, France). 1H-, 13C-NMR and MALDI-TOF (2,5-dihydroxybenzoic acid matrix) analyses were performed according to procedures previously described in the literature [14].
3.2. Synthesis of COSamf 1
Chitosan (2.1g, 13 mmole of GlcN unit) was solubilized in 100 mL of water by addition of 1.2 mL HCl (37% w/w). A freshly prepared 5 mL aqueous solution of NaNO2 (90 mg, 0.13 mmol for GlcN/NaNO2 molar ratio = 10:1) was added and the reaction was allowed to proceed for 12 h at room temperature. Oligomers were precipitated by addition of ammonium hydroxide solution (28% w/w) to pH ~9, washed several times with distilled water until neutral pH, then freeze-dried leading to COSamf 1 (1.6 g, 76% mass yield) as a white powder. 1H-NMR (500 MHz, D2O): δ (ppm) 5.10 (d, J = 5.4 Hz, 1H, H-1 amf), 4.90–4.70 (m, 22H, H-1 GlcN), 4.45 (t, J = 4.9 Hz, 1H, H-3 amf), 4.23 (t, J = 4.9 Hz, 1H, H-4 amf), 4.13 (m, 1H, H-5 amf), 4.05–3.45 (m, 113H, H-2 and H-6 amf, H-3 to H-6 GlcN), 3.20–3.10 (m, 22H, H-2 GlcN). 13C-NMR (125 MHz, D2O): δ (ppm) 99.2 (C-1′ GlcN), 98.6 (C-1 GlcN), 89.8 (C-1 amf), 86.5 (C-4 amf), 85.6 (C-2 amf), 82.6 (C-5 amf), 77.2 (C-3 amf), 77.0 (C-4 GlcN), 76.9 (C-5′ GlcN), 75.3 (C-5 GlcN), 72.5 (C-3′ GlcN), 71.0 (C-3 GlcN), 70.2 (C-4′ GlcN), 61.4 (C-6 amf), 60.9 (C-6′ GlcN), 60.6 (C-6 GlcN), 56.5 (C-2 GlcN), 56.2 (C-2′ GlcN). Note that C′ represents the carbon atoms of the GlcN unit linked to the amf unit. MALDI-TOF MS (positive reflectron mode): major peak at m/z 1473.9 assigned to HO-(GlcN)8-amf (m/z monoisotopic calcd for [C54H98O37N8Na]+ = 1473.6 m/z).
3.3. Synthesis of Octanoic Hydrazide-Linked Chitooligosaccharide-2,5-Anhydro-d-Mannofuranose 2
COSamf 1 (1g, 0.3 mmol of amf unit) was dissolved into 20 mL aqueous ammonium acetate buffer (0.2 M, pH 4.5). 600 mg of octanoic hydrazide (3.0 mmol, 10 eq./amf unit) solubilized in 20 mL of ethanol were added and the mixture was stirred for one day at room temperature. Sodium cyanoborohydride (190 mg, 3.0 mmol) was then added and the mixture was stirred for one day at room temperature. Oligomers were precipitated by addition of ammonium hydroxide solution (28% w/w) to pH ~9, washed several times with distilled water until neutral pH, then freeze-dried leading to the compound 2 (0.9 g, 90% mass yield) as white powder. 1H-NMR (500 MHz, D2O): δ(ppm) 4.91 (m, 20H, H-1 GlcN), 4.32 (m, 1H, H-3 amf), 4.25–4.10 (m, 3H, H-2 amf, H-4 amf, H-5 amf), 4.05–3.50 (m, 122H, H-3 to H-6 GlcN, H-6 amf), 3.37 (m, 2H, CH2N), 3.25–3.10 (m, 20H, H-2 GlcN), 2.30 (m, 2H, CH2CO), 1.60 (m, 2H, CH2), 1.35–1.20 (m, 8H, 4 × CH2), 0.85 (m, 3H, CH3). 13C-NMR (125 MHz, D2O): δ(ppm), 175.5 (CO), 99.3 (C-1′ GlcN), 98.2 (C-1 GlcN), 86.6 (C-4 amf), 82.9 (C-5 amf), 79.3 (C-2 amf), 78.0 (C-3 amf), 77.1 (C-5′ GlcN), 77.0 (C-4 GlcN), 75.4 (C-5 GlcN), 72.4 (C-3′ GlcN), 70.7 (C-3 GlcN), 70.3 (C-4′ GlcN), 61.9 (C-6 amf), 61.0 (C-6′ GlcN), 60.7 (C-6 GlcN), 56.5 (C-2 GlcN), 56.2 (C-2′ GlcN), 52.3 (CH2N), 34.0, 31.6, 28.7, 28.6, 25.3, 22.6 (6 × CH2), 14.1 (CH3). Note that C′ represents carbon atoms of the GlcN unit linked to the amf unit. MALDI-TOF MS (positive reflectron mode): major peak at 1777.2 m/z assigned to HO-(GlcN)9-C14H28O5N2 (m/z monoisotopic calcd for [C68H127O41N11Na]+ = 1776.8 m/z).
Supplementary Materials
Supplementary materials can be accessed at: http://www.mdpi.com/1422-8599/2016/3/M904.
Supplementary File 1Supplementary File 2Supplementary File 3Acknowledgments
The authors thank Catherine Ladavière (IMP, CNRS) and Bernard Fenet (CCRMN, Université Lyon 1) for their helpful assistance and discussions in MALDI-TOF mass spectrometry and NMR spectroscopy analyses, respectively.
Author Contributions
S.T. conceived and designed the experiments; A.M. performed the experiments; A.M. and S.T. analyzed the data; S.T. wrote the paper.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kurita, K. Chitin and chitosan: Functional biopolymers from marine crustaceans. Mar. Biotechnol. 2006, 8, 203–226. [Google Scholar] [CrossRef] [PubMed]
- Dash, M.; Chiellini, F.; Ottenbrite, R.M.; Chiellini, E. Chitosan: A versatile semi-synthetic polymer in biomedical applications. Prog. Polym. Sci. 2011, 36, 981–1014. [Google Scholar] [CrossRef]
- Younes, I.; Rinaudo, M. Chitin and chitosan preparation from marine sources. Structure, properties and applications. Mar. Drugs 2015, 13, 1133–1174. [Google Scholar] [CrossRef] [PubMed]
- Hamed, I.; Ozogul, F.; Regenstein, J.M. Industrial applications of crustacean by-products (chitin, chitosan and chitooligosaccharides): A review. Trends Food Sci. Technol. 2016, 48, 40–50. [Google Scholar] [CrossRef]
- Mourya, V.K.; Inamdar, N.N.; Choudhari, Y.M. Chitooligosaccharides: Synthesis, characterization and applications. Polym. Sci. Ser. A 2011, 53, 583–612. [Google Scholar] [CrossRef]
- Kim, S.-K.; Rajapakse, N. Enzymatic production and biological activities of chitosan oligosaccharides (COS): A review. Carbohydr. Polym. 2005, 62, 357–368. [Google Scholar] [CrossRef]
- Aam, B.B.; Heggset, E.B.; Norberg, A.L.; Sørlie, M.; Vårum, K.M.; Eijsink, V.G.H. Production of chitooligosaccharides and their potential applications in medicine. Mar. Drugs 2010, 8, 1482–1517. [Google Scholar] [CrossRef] [PubMed]
- Xia, W.; Liu, P.; Zhang, J.; Chen, J. Biological activities of chitosan and chitooligosaccharides. Food Hydrocoll. 2011, 25, 170–179. [Google Scholar] [CrossRef]
- Hussain, I.; Singh, T.; Chittenden, C. Preparation of chitosan oligomers and characterization: Their antifungal activities and decay resistance. Holzforschung 2012, 66, 119–125. [Google Scholar] [CrossRef]
- Das, S.N.; Madhuprakasha, J.; Sarma, P.V.S.R.N.; Purushotham, P.; Suma, K.; Manjeet, K.; Rambabu, S.; el Gueddari, N.E.; Moerschbacher, B.M.; Podile, A.R. Biotechnological approaches for field applications of chitooligosaccharides (COS) to induce innate immunity in plants. Crit. Rev. Biotechnol. 2015, 35, 29–43. [Google Scholar] [CrossRef] [PubMed]
- Lodhi, G.; Kim, Y.-S.; Hwang, J.-W.; Kim, S.-K.; Jeon, Y.-J.; Je, J.-Y.; Ahn, C.-B.; Moon, S.-H.; Jeon, B.-T.; Park, P.-J. Chitooligosaccharide and its derivatives: Preparation and biological applications. BioMed. Res. Int. 2014. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, M.O.; Hogg, B.; Bono, J.-J.; Samain, E.; Driguez, H. New access to lipo-chitooligosaccharides nodulations factors. Org. Biomol. Chem. 2004, 2, 1908–1910. [Google Scholar] [CrossRef] [PubMed]
- Illy, N.; Robitzer, M.; Auvergne, R.; Caillol, S.; David, G.; Boutevin, B. Synthesis of water-soluble allyl-functionalized oligochitosan and its modification by thiol-ene addition in water. J. Polym. Sci. Part A Polym. Chem. 2014, 52, 39–48. [Google Scholar] [CrossRef]
- Trombotto, S.; Ladavière, C.; Delolme, F.; Domard, A. Chemical preparation and structural characterization of a homogeneous series of chitin/chitosan oligomers. Biomacromolecules 2008, 9, 1731–1738. [Google Scholar] [CrossRef] [PubMed]
- Abla, M.; Marmuse, L.; Delolme, F.; Vors, J.-P.; Ladavière, C.; Trombotto, S. Access to tetra-N-acetyl-chitopentaose by chemical N-acetylation of glucosamine pentamer. Carbohydr. Polym. 2013, 98, 770–777. [Google Scholar] [CrossRef] [PubMed]
- Tommeraas, K.; Varum, K.M.; Christensen, B.E.; Smidsrod, O. Preparation and characterization of oligosaccharides produced by nitrous acid depolymerization of chitosans. Carbohydr. Res. 2001, 333, 137–144. [Google Scholar] [CrossRef]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).