Abstract
The compound named in the title was prepared from N1,N4-diphenethylterephthalamide 1. The resulting bis terephthalamide was subjected to an intramolecular α-amidoalkylation reaction with paraformaldehyde in the presence of heterogeneous catalyst TfOH/SiO2 to obtain 1,4-phenylenebis[(6,7-dimethoxy-3,4-dihydroisoquinolin-2(1H)-yl)methanone]. The structure of the newly synthesized compound was determined using 1H, 13C-NMR, UV, IR and mass spectral data.
1. Introduction
The alkaloids containing an isoquinoline skeleton are one of the largest classes of plant alkaloids which display a broad spectrum of biological activities [1]. In recent years, acid catalysts have increasingly found use as catalytic systems of acid absorbed on silica [2]. In addition to promoting a successful reaction, their ability to be recovered and consistently reused in subsequent reactions identifies them as environmentally friendly “green reagents“[3]. Based on our previous experience of the application of intramolecular α-amidoalkylation for the synthesis of isoquinoline compounds, we have successfully obtained 1,4-phenylenebis[(6,7-dimethoxy-3,4-dihydroisoquinolin-2(1H)-yl)methanone] using triflic acid absorbed on silica, as a green catalyst, in an intramolecular α-amidoalkylation reaction.
2. Results
In this work we report the successful synthesis of 1,4-phenylenebis[(6,7-dimethoxy-3,4-dihydroisoquinolin-2(1H)-yl)methanone] as shown in Scheme 1.
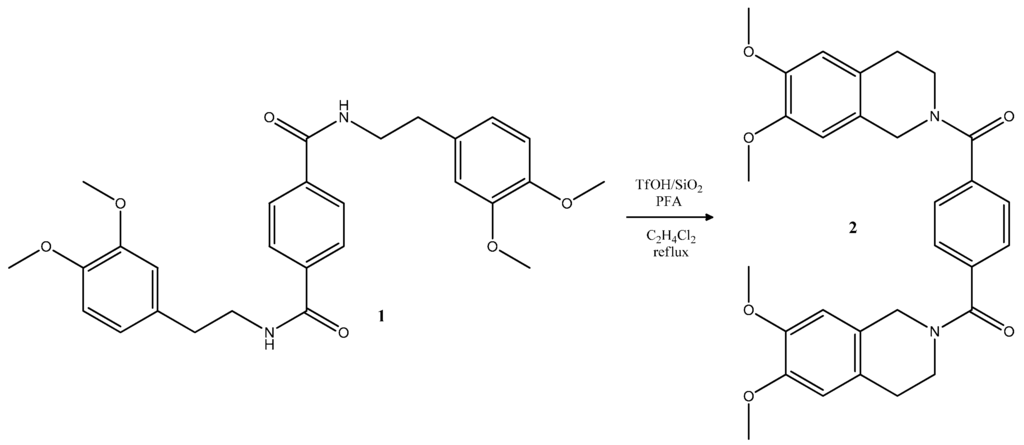
Scheme 1.
Synthesis of 1,4-phenylenebis[(6,7-dimethoxy-3,4-dihydroisoquinolin-2(1H)-yl)methanone] 2.
The starting amide 1 was synthesized through the application of the Saidov [4] synthetic procedure. Based on our previous experience [5], initial cyclization reactions were performed for amide 1 (Scheme 1) using paraformaldehyde in dichloroethane in the presence of the catalytic system of acid absorbed on silica. In our previous experiments we have reported the use of heterogeneous catalytic systems of polyphosphoric acid absorbed on silica as a new “green” agent for the synthesis of Cherylline derivatives [6]. In search of other new “green” agents, we studied the possibility of applying a TfOH/SiO2 system as a heterogeneous acid catalyst in an intramolecular α-amidoalkylation reaction. Triflic acid (TfOH) is termed a “super acid”, as it is perhaps one of the most versatile Brønsted acid catalysts that can be used in a vast array of organic reactions. Because of its high corrosiveness and due to the fact that it is a fuming liquid, difficulties remain in storage, transportation, handling and waste disposal that severely restrict their application in industry [7].
For the cyclization step, to amide 1 (Scheme 1) dissolved in dichloroethane we added paraformaldehyde in excess and TfOH/SiO2. Compound 2 was successfully obtained by refluxing the reaction mixture for 10 min. After completion of the reaction, the reaction mixture was cooled, and the catalyst was removed via filtration. The solvent was then distilled via rotary evaporator.
Compound 2 was obtained as a light yellow solid (93% yield) with a melting point recorded at 199–202 °C. The UV spectrum exhibited absorption maxima λmax 203, 234 and 284 nm. The mass of the compound in the HRMS spectrum was found at [M + Na]+, m/z = 539.2128 (Calculated: 539.2158). The signals in the NMR spectrums are doubled due to rotamers in nearly a 1:1 ratio.
3. Materials and Methods
All the reagents and chemicals were purchased from commercial sources (Sigma-Aldrich, Sofia, Bulgaria) and used as received. Melting points were determined on a Boetius hot stage apparatus and are uncorrected. The spectral data were recorded on a Bruker Avance II + 600 spectrometer (BAS-IOCCP—Sofia, Sofia, Bulgaria). The 1H-NMR and 13C-NMR spectra for compound 2 were taken in DMSO at 600 MHz and 150.9 MHz, respectively. Chemical shifts are given in ppm relative and were referenced to TMS (δ = 0.00 ppm) as an internal standard with the coupling constants indicated in Hz. The NMR spectra were taken at room temperature (ac. 295 K). TLC was carried out on precoated 0.2 mm Fluka silica gel 60 plates, using diethyl ether/n-hexane = 1/1 as chromatographic system. The catalyst TfOH/SiO2 was prepared using the procedure detailed by Liu et al. [3].
Synthesis of 1,4-Phenylenebis[(6,7-dimethoxy-3,4-dihydroisoquinolin-2(1H)-yl)methanone] 2
To a solution of N1,N4-diphenethylterephthalamide 1 (3 mmol) and paraformaldehyde (5 mmol) in C2H4Cl2 (10 mL) was added 0.06 g of catalyst (TfOH/SiO2, 0.5 mmol/g). The reaction mixture was placed under reflux for 10 min. After the completion of the reaction, the reaction mixture was cooled and the catalyst was separated by simple filtration.
1,4-Phenylenebis[(6,7-dimethoxy-3,4-dihydroisoquinolin-2(1H)-yl)methanone] (2). White solid (M.p. 199–202 °C). 1H-NMR (600 MHz, DMSO) δ ppm 2.71 (s, 2H), 2.85 (s, 2H), 3.55 (m, 2H), 3.73–3.81 (m, 12H), 3.93 (m, 2H), 4.41 (s, 2H), 4.77 (s, 2H), 6.33 (s, 1H), 6.56–6.61 (m, 3H), 7.44 (s, 4H); 13C-NMR (150.9 MHz, DMSO) δ ppm 146.9, 136.6, 126.4, 126.3, 110.5, 110.3, 108.2, 107.5, 54.9, 48.5, 44.3, 42.5. λmax, MeOH: 203, 234, 284 nm. HRMS (ESI) m/z calcd for C30H32N2O6 [M + Na]+ = 539.2158, found 539.2128. IR (KBr) νmax., cm−1: 760γ(Csp2-H), 847γ(Csp2-H), 1037νs(Csp2-O-C), 1207νas(Csp2-O-C), 1442δ(R2=N-CH2-), 1518ν(Csp2-Csp2), 1631γ(N-C=O), 2835δas(OCH2-H), 2933νas(CH2), 3067ν(Csp2-H).
Copies of all spectra and ESI-HRMS (Figures S1–S5) are provided in the Supplementary Materials.
Supplementary materials
The molefiles and the other supplementary materials can be found at http://www.mdpi.com/1422-8599/2016/3/M902.
Supplementary File 1Supplementary File 2Supplementary File 3Supplementary File 4Acknowledgments
We acknowledge financial support from the fund for scientific research of the University of Plovdiv—НИ15-ХФ-001.
Author Contributions
Both authors contributed equally to both the experimental and writing work.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kamlah, A.; Lirk, F.; Bracher, F. A new approach to 1-substituted β-carbolines and isoquinolines utilizing tributyl[(Z)-2-ethoxyvinyl]stannane as a C-3, C-4 building block. Tetrahedron 2016, 72, 837–845. [Google Scholar] [CrossRef]
- Kaur, M.; Sharma, S.; Bedi, P.M.S. Silica supported Brönsted acids as catalyst in organic transformations: A comprehensive review. Chin. J. Catal. 2015, 36, 520–549. [Google Scholar] [CrossRef]
- Liu, P.N.; Xia, F.; Wang, Q.W.; Ren, Y.J.; Chen, J.Q. Triflic acid adsorbed on silica gel as an efficient and recyclable catalyst for the addition of β-dicarbonyl compounds to alcohols and alkenes. Green Chem. 2010, 12, 1049–1055. [Google Scholar] [CrossRef]
- Saidov, A.S.; Levkovich, M.G.; Alimova, M.; Vinogradova, V.I. Synthesis of bis-tetrahydroisoquinolines based on homoveratrylamine and dibasic acids. 2. Chem. Nat. Compd. 2014, 49, 1099–1104. [Google Scholar] [CrossRef]
- Venkov, A.; Temnyalova, T.; Ivanov, I. Synthesis of N,N’-bis(1,2,3,4-tetrahydroisoquinolinyl) dicarboxamides by an intramolecular α-diamidoalkylation reaction. Synth. Commun. 1995, 25, 1419–1425. [Google Scholar] [CrossRef]
- Manolov, S.; Nikolova, S.; Ivanov, I. Silica-supported polyphosphoric acid in the synthesis of 4-substituted tetrahydroisoquinoline derivatives. Molecules 2013, 18, 1869–1880. [Google Scholar] [CrossRef] [PubMed]
- Kumar, D.; Sonawane, M.; Pujala, B.; Jain, V.K.; Bhagat, S.; Chakraborti, A.K. Supported protic acid-catalyzed synthesis of 2,3-disubstituted thiazolidin-4-ones: Enhancement of the catalytic potential of protic acid by adsorption on solid supports. Green Chem. 2013, 15, 2872–2884. [Google Scholar] [CrossRef]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).