Abstract
A new sterically hindered isoindoline-based 1,3-bis(5′-methyl-4′-phenyl-2′-thiazolylimino)isoindoline (1) was synthesized by fusion method with satisfyingly good yield. The structure of the newly synthesized compound was identified by FT-IR, UV-Vis, 1H-NMR, 13C-NMR and X-ray analysis.
1. Introduction
The tridentate bis(arylimino)isoindolines (BAI) belong to the class of pincer type ligands. They are considered potent metal chelators and their coordination chemistry is extensively studied as well. The ligand family originates from diiminoisoindolines and phthalocyanines that are building blocks of supramolecular architectures. It has been proven in a couple of biological studies that they may possess antiproliferative activity and they can act as diuretic or blood pressure regulator agents [1]. BAI with thiazole arms can add an extra function to transition metal complexes they coordinate to. Metal ions such as Cu, Fe, Mn, Co, Ni, etc. approach the N atom of the thiazole, while conditions may be altered in order to achieve S-coordination for Pd (or Pt) [2].
Bulky aliphatic or aromatic functional groups on the thiazole arm can easily block homoleptic complex formation that is characteristic for isoindoline complexes [1]. This can create an advantageous situation for cyclometallation [3] or intramolecular oxidative CH bond (benzylic and/or aromatic) activation reactions via peroxo or high valent oxo species [4].
2. Results and Discussion
BAIs are generally synthesized by Siegl’s [5] method or Linstead’s [6] method. This involves a direct and an indirect procedure respectively from the starting material phthalonitrile. The third option is a fusion synthesis also known from the literature, however, it is less commonly used [7]. Fusing phthalonitrile with primary amines requires high temperature (180–190 °C) but there are advantages like shorter reaction time and solvent-free conditions (Scheme 1). The addition of a primary amine to phthalonitrile results in an intramolecular cyclization to form a relatively stable 1-arylimino-3-iminoisoindoline, which may undergo addition of a second amine with loss of ammonia and formation of the BAI via nucleophilic addition reaction [8].
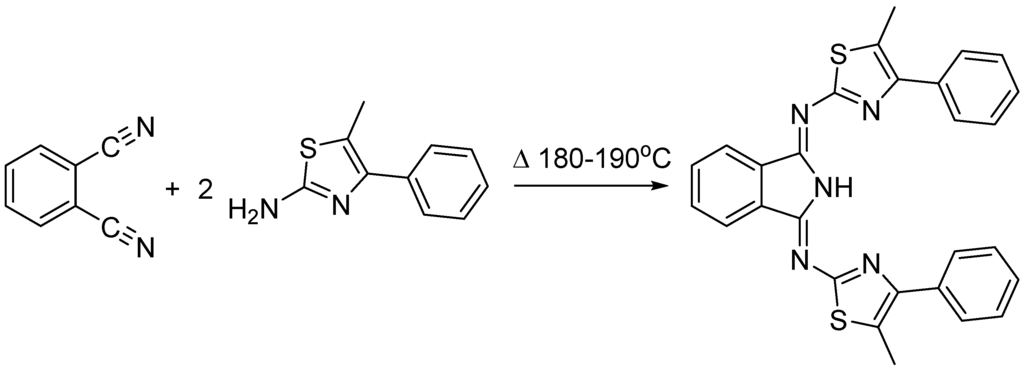
Scheme 1.
Fusion synthesis of 1.
The preparation of the title compound (1) has been carried out accordingly, yielding 80% orange product. The single crystal X-ray structure of 1 shows a protonated central N atom with N3-H3 = 0.836 Å distance. The imino arms on the isoindoline core are nearly symmetrical (C1-N2-C9 = 119.31° and C8-N4-C19 = 118.97°), only the thiazole moieties are out of plane due to steric forces.
3. Experimental Section
3.1. General Methods, Analytical and Physical Measurements
The crystal evaluation and intensity data collection for 1 were performed with a Bruker-Nonius Kappa CCD single-crystal diffractometer with Mo-Kα radiation (λ = 0.71073 Å) at 293(2). Crystallographic data and selected bond lengths and angles are listed in Table S1. Microanalyses were done by the Microanalytical Service of the University of Pannonia. Infrared spectra were recorded on an Avatar 330 FT-IR Thermo Nicolet instrument (Thermo Nicolet Corporation, Madison, WI, USA) using samples mulled in KBr pellets. UV-Vis spectra were recorded on a Cary 60 spectrophotometer (Agilent Technologies, Penang, Malaysia) equipped with a fiber-optic probe with 1 cm path length. NMR spectrum was recorded on a Bruker Avance 400 spectrometer (Bruker Biospin AG, Fällanden, Switzerland).
3.2. Synthesis of 1,3-Bis(5′-methyl-4′-phenyl-2′-thiazolylimino)isoindoline (1)
A solvent-free method has been applied for the synthesis of (1) (Figure 1). Phthalonitrile (1.20 g, 9.4 mmol) and 2-amino-5-methyl-4-phenylthiazole (3.58 g, 18.8 mmol) were fused at high temperature (190 °C) that was maintained until ammonia evolution was observed. The solid material that formed on cooling was dissolved in methanol and crystallized by slow evaporation of the solvent resulting orange needle shaped crystals.
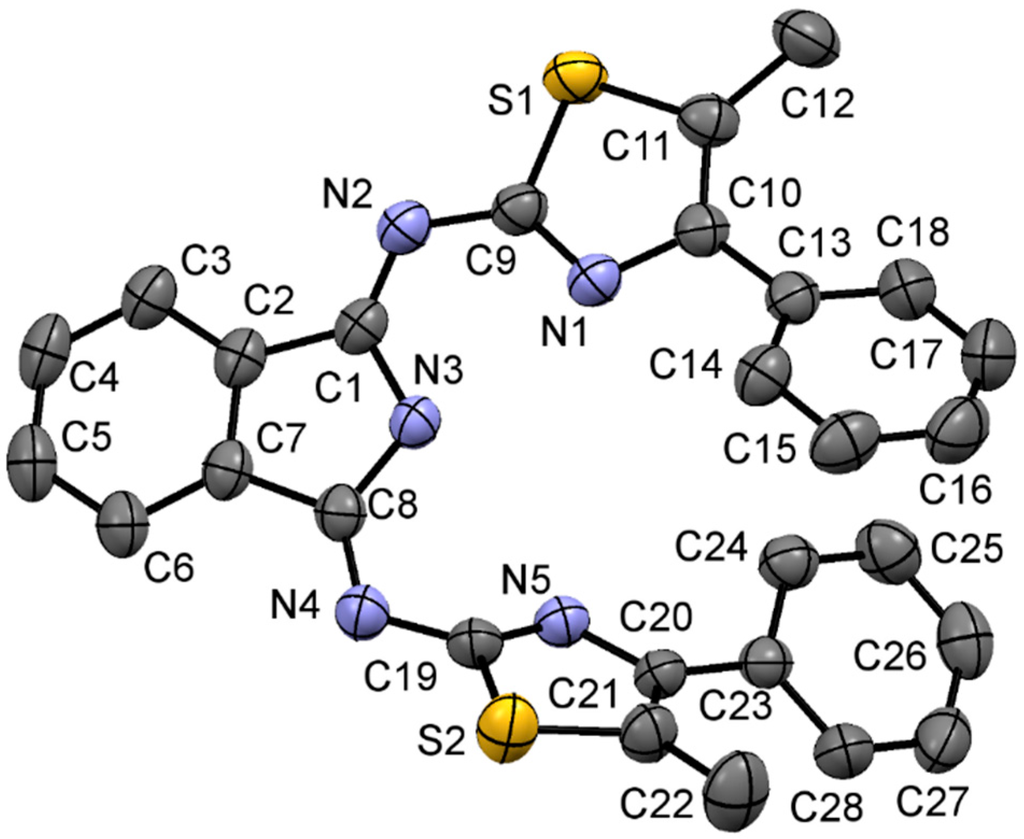
Figure 1.
X-ray structure of 1.
1,3-Bis(5′-methyl-4′-phenyl-2′-thiazolylimino)isoindoline: orange crystal. IR (KBr, cm−1) (Figure S3): 3252 w, 3048 w, 2937 w, 2913 w, 2856 w, 1640 w, 1613 vs, 1473 m, 1437 m, 1297 m, 1216 s, 1162 m, 1098 w, 1048 m, 1002 w, 875 m, 772 s, 700 s, 656 m, 598 w, 567 w, 495 w, 471 w. UV–Vis (DMF) λmax(log ε, dm3mol−1cm−1): 279 (4.65), 286 (4.64), 388 (4.35), 450 (4.36), 504 (4.06). 1H-NMR (400 MHz, CDCl3) (Figure S1): 12.37 (s, 1 H), 8.06–8.02 (m, 2 H), 7.67–7.62 (m, 2 H), 7.56–7.51 (m, 4 H), 7.1–7.10 (m, 6 H), 2.52 (s, 6 H). 13C-NMR (100 MHz, CDCl3) δ (ppm) (Figure S2): 13.31, 123.1, 127.2, 127.6, 128.2, 128.5, 132.2, 134.5, 135.1, 149.4, 151.8, 166.1. C28H21N5S2 (491.64): calcd. C 68.41, H 4.31, N 14.25; found C 68.39, H 4.32, N 14.27.
Supplementary Materials
Supplementary File 1Supplementary File 2Supplementary File 3Supplementary File 4CCDC 1441356 contains the supplementary crystallographic data for this article and can be obtained free of charge from The Cambridge Crystallographic Data Centre via http://www.ccdc.cam.ac.uk/data_request/cif. 1H-NMR, 13C-NMR and IR spectra are reported in the supplementary materials as Figure S1–S3 together with crystal data and structure refinement parameters as Table S1. They and the molfiles can be found at http://www.mdpi.com/1422-8599/2016/1/M882.
Acknowledgments
Financial support of the Hungarian National Research Fund (OTKA K108489), COST CM1003, and the János Bolyai Research Scholarship of the Hungarian Academy of Sciences are gratefully acknowledged.
Author Contributions
Robert Csonka: writing of manuscript, IR, NMR interpretation, literature search; Miklós István Szávuly: synthetic work; Michel Giorgi: X-ray crystallography; Gábor Speier and József Kaizer: design of synthesis, literature search.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Csonka, R.; Speier, G.; Kaizer, J. Isoindoline-derived ligands and applications. RSC Adv. 2015, 5, 18401–18419. [Google Scholar] [CrossRef]
- Bröring, M.; Kleeberg, C. Facile intramolecular C(sp3)–H bond activation with PdII. Chem. Commun. 2008, 2777–2778. [Google Scholar] [CrossRef] [PubMed]
- Bröring, M.; Kleeberg, C. Cyclometalation vs. Werner-type coordination of sterically enforced palladium(II)-1,3-bis(pyridyl-2-imino)isoindolines (Pd-BPIs). Dalton Trans. 2007, 1101–1103. [Google Scholar] [CrossRef] [PubMed]
- Pap, J.S.; Cranswick, M.A.; Balogh-Hergovich, É.; Baráth, G.; Giorgi, M.; Rohde, G.T.; Kaizer, J.; Speier, G.; Que, L., Jr. An Iron(II)[1,3-bis(2′-pyridylimino)isoindoline] Complex as a Catalyst for Substrate Oxidation with H2O2—Evidence for a Transient Peroxidodiiron(III) Species. Eur. J. Inorg. Chem. 2013, 3858–3866. [Google Scholar] [CrossRef] [PubMed]
- Siegl, W.O. A new bis-cheiating ligand system. Synthesis and chelating behavior. Inorg. Chim. Acta 1977, 25, L65–L66. [Google Scholar] [CrossRef]
- Elvidge, J.A.; Linstead, R.P. Heterocyclic Imines. Part I. Imino-derivatives of isoindoline and their reaction with primary bases. J. Chem. Soc. 1952, 5000–5007. [Google Scholar] [CrossRef]
- Domaille, P.J.; Harlow, R.L.; Ittel, S.D.; Peet, W.G. NMR spectra of paramagnetic group 8 complexes of bis(pyridy1imino)isoindoline. Inorg. Chem. 1983, 22, 3944–3952. [Google Scholar] [CrossRef]
- Siegl, W.O. Metal Ion Activation of Nitriles. Syntheses of 1,3-bis( arylimino)isoindolines. J. Org. Chem. 1977, 42, 1872–1878. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).