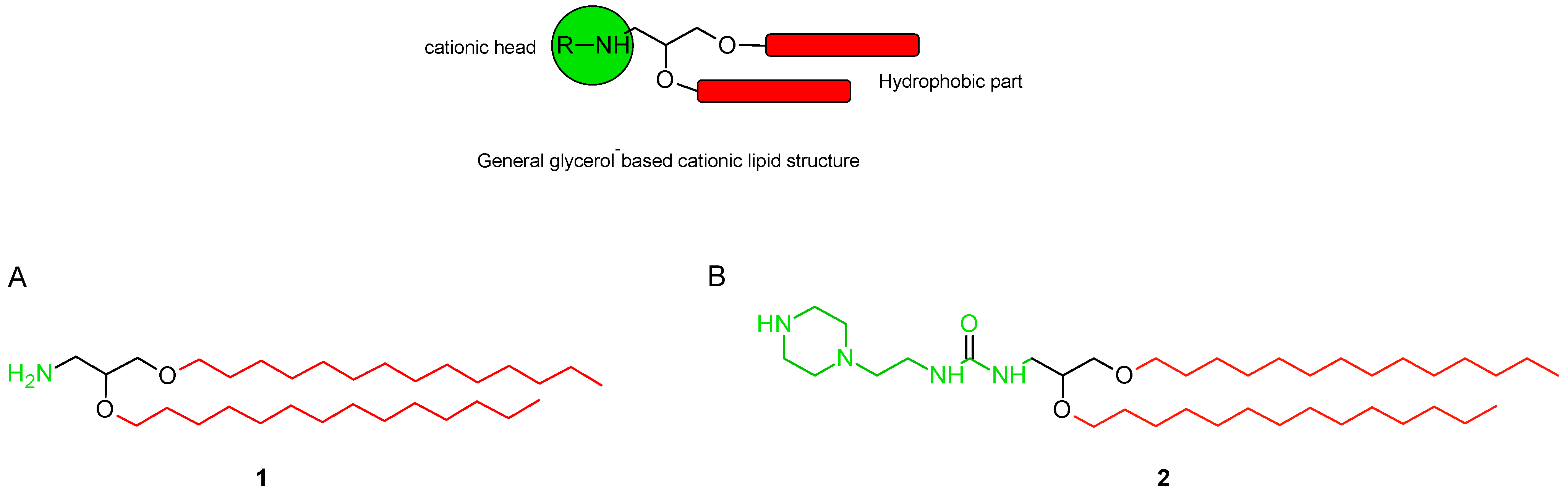
1-[2,3-Bis(tetradecyloxy)propyl]-3-[2-(piperazin-1-yl)ethyl]urea
Abstract
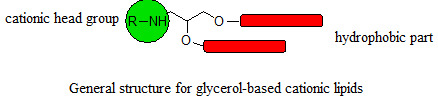
:1. Introduction
2. Results and Discussion
3. Experimental Section
3.1. General Methods
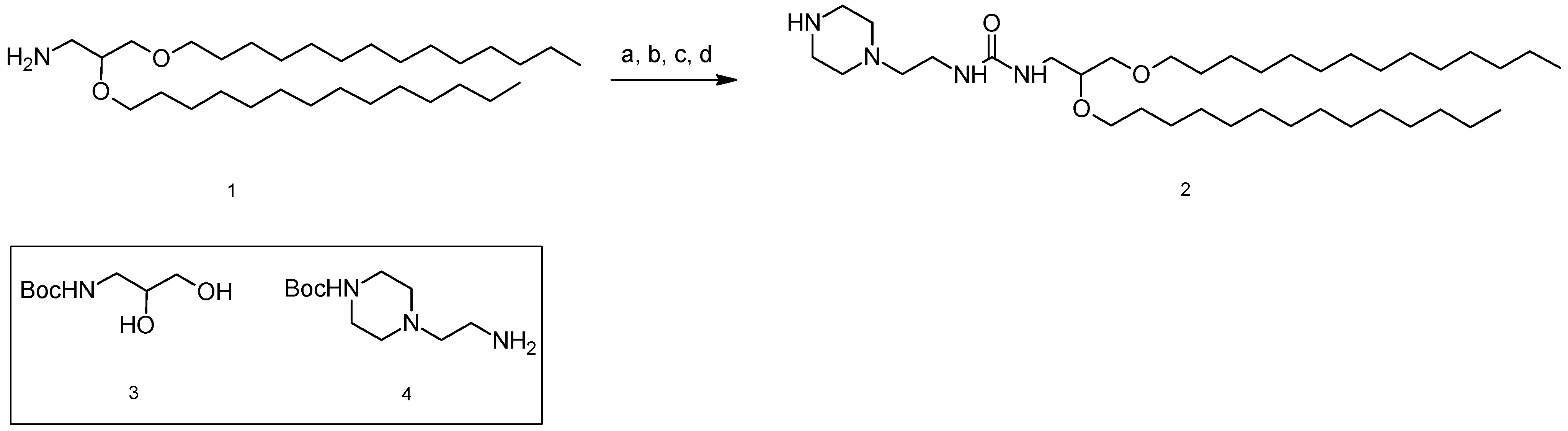
3.2. 1-[2,3-Bis(tetradecyloxy)propyl]-3-[2-(piperazin-1-yl)ethyl]urea (2)
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3Supplementary File 4Acknowledgments
Author Contributions
Conflicts of Interest
References
- Won, Y.-W.; Lim, K.S.; Kim, Y.-H. Intracellular organelle-targeted non-viral gene delivery systems. J. Control. Release 2011, 152, 99–109. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.; Zhang, S.-B.; Wang, B.; Yang, B.-L.; Zhi, D.-F. The biological routes of gene delivery mediated by lipid-based non-viral vectors. Exp. Opin. Drug Deliv. 2009, 6, 1351–1361. [Google Scholar] [CrossRef] [PubMed]
- Marvaniya, H.M.; Parikh, P.K.; Patel, V.R.; Modi, K.N.; Sen, D.J. Dendrimer nanocarriers as versatile vectors in gene delivery. J. Chem. Pharm. Res. 2010, 2, 97–108. [Google Scholar]
- Lehto, T.; Kurrikoff, K.; Langel, U. Cell-penetrating peptides for the delivery of nucleic acids. Exp. Opin. Drug Deliv. 2012, 9, 823–836. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.J.; Kim, S.W. Efficient siRNA delivery with non-viral polymeric vehicles. Pharm. Res. 2009, 26, 657–666. [Google Scholar] [CrossRef] [PubMed]
- Felgner, P.L.; Gadek, T.R.; Holm, M.; Roman, R.; Chan, H.W.; Wenz, M.; Northrop, J.P.; Ringold, G.M.; Danielsen, M. Lipofection: A highly efficient, lipid-mediated DNA-transfection procedure. Proc. Natl. Acad. Sci. USA 1987, 84, 7413–7417. [Google Scholar] [CrossRef] [PubMed]
- Zhi, D.; Zhang, S.; Cui, S.; Zhao, Y.; Wang, Y.; Zhao, D. The headgroup evolution of cationic lipids for gene delivery. Bioconjug. Chem. 2013, 24, 487–519. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Wang, F.; Wu, Y.; Davidson, G.; Levkin, P.A. Combinatorial synthesis and high-throughput screening of alkyl amines for nonviral gene delivery. Bioconjug. Chem. 2013, 24, 1543–1551. [Google Scholar] [CrossRef] [PubMed]
- Akinc, A.; Zumbuehl, A.; Goldberg, M.; Leshchiner, E.S.; Busini, V.; Hossain, N.; Bacallado, S.A.; Nguyen, D.N.; Fuller, J.; Alvarez, R.; et al. A combinatorial library of lipid-like materials for delivery of RNAi therapeutics. Nat. Biotechnol. 2008, 26, 561–569. [Google Scholar] [CrossRef] [PubMed]
- Puras, G.; Zárate, J.; Agirre, M.; Díaz-Tahoces, A.; Avilés-Trigueros, M.; Grijalvo, S.; Eritja, R.; Fenández, E.; Pedraz, J.L. A novel formulation based on 2,3-di(tetradecyloxy)propan-1-amine cationic lipid combined with polysorbate 80 for efficient gene delivery to the retina. Pharm. Res. 2014, 31, 1665–1675. [Google Scholar]
- Puras, G.; Mashal, M.; Zárate, J.; Agirre, M.; Ojeda, E.; Grijalvo, S.; Eritja, R.; Díaz-Tahoces, A.; Martínez Navarrete, G.; Avilés-Trigueros, M.; et al. A novel cationic noisome formulation for gene delivery to the retina. J. Control. Release 2014, 174, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Baxendale, I.R.; Brusotti, G.; Matsuoka, M.; Ley, S.V. Synthesis of nornicotine, nicotine and other functionalized derivatives using solid-supported reagents and scavengers. J. Chem. Soc., Perkin Trans. 1 2002, 143–154. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grijalvo, S.; Núñez, S.; Eritja, R. 1-[2,3-Bis(tetradecyloxy)propyl]-3-[2-(piperazin-1-yl)ethyl]urea. Molbank 2015, 2015, M873. https://doi.org/10.3390/M873
Grijalvo S, Núñez S, Eritja R. 1-[2,3-Bis(tetradecyloxy)propyl]-3-[2-(piperazin-1-yl)ethyl]urea. Molbank. 2015; 2015(4):M873. https://doi.org/10.3390/M873
Chicago/Turabian StyleGrijalvo, Santiago, Samuel Núñez, and Ramon Eritja. 2015. "1-[2,3-Bis(tetradecyloxy)propyl]-3-[2-(piperazin-1-yl)ethyl]urea" Molbank 2015, no. 4: M873. https://doi.org/10.3390/M873
APA StyleGrijalvo, S., Núñez, S., & Eritja, R. (2015). 1-[2,3-Bis(tetradecyloxy)propyl]-3-[2-(piperazin-1-yl)ethyl]urea. Molbank, 2015(4), M873. https://doi.org/10.3390/M873