Abstract
We report a novel protocol for the synthesis of 3-(3,4-dihydroxyphenyl)-8-hydroxy-2H-chromen-2-one via demethylation/delactonization/elimination/lactonization/decarboxylation domino reaction sequence of diastereomeric mixture of cis- and trans-3-(2,3-dimethoxyphenyl)-6,7-dimethoxy-1-oxoisochroman-4-carboxylic acids in boiling HBr/AcOH. The structure of the target compound was established for the first time by means of spectral methods such as 1H-, 13C-, DEPT-135-NMR, IR and HRMS.
1. Introduction
Coumarins are a large class of oxygenated heterocyclic secondary metabolites that are biosynthesized by plants and fruits de novo [1] as a defense response to stress (drought and cold), wound, viral infection or invasion by bacterial or fungal pathogens [2,3]. Natural 3-arylcoumarins are known to display a wide range of biological activities [4,5,6,7], such as anti-inflammatory, anticoagulant, anticancer, vasorelaxant, and antiviral, to name just a few. However, it is noteworthy that coumarins are produced only at a nanogram scale in plants, thus showing the need for the development of methods for their rational synthesis. In this regards, herein we report a novel protocol for the facile synthesis of polyhydroxylated 3-arylcoumarin from 3-(2-methoxyphenyl)-1-oxoisochroman-4-carboxylic acids in the presence of hydrobromic acid.
2. Results and Discussion
It is a well-known fact, that domino reactions are a powerful bond-forming tool that allows the preparation of complex molecules in a one-pot sequential and efficient manner. In a recent our articles, we demonstrated that polyhydroxylated cis-restricted stilbenes [8,9] or 3-arylcoumarins (2) bearing carboxylic function in their structure [10] can be obtained via one-pot procedure from diastereomeric mixture of methoxylated cis- and trans-3-aryl-3,4-dihydroisocoumarin-4-carboxylic acids [11] by treatment with BBr3. However, just recently we observed that replacement of BBr3 with HBr as a demethylating agent leads to a subsequent reaction, which produces decarboxylated 3-arylcoumarin derivative—the title compound 3 (Scheme 1). Its synthesis from 2-(2,3-dimethoxyphenyl)-1-(3,4-dimethoxyphenyl)acrylonitrile in neat pyridine hydrochloride had been previously reported elsewhere [12], but detailed experimental procedure, as well as spectral data from 1H- and 13C-NMR analyses were not provided. In this regard, herein we report a novel approach for the synthesis of 3 via domino transformation (cf. Scheme 2) consisting of five sequential steps, including demethylation, lactone ring opening, elimination, isomerization, lactone ring closure and decarboxylation reactions. The main part of this mechanism was deduced on the base of our previous experiments (for details see [10]) and the starting diastereomeric mixture of cis- and trans-3-(2,3-dimethoxyphenyl)-6,7-dimethoxy-1-oxoisochroman-4-carboxylic acids (1) was obtained in 10 min from 6,7-dimetoxihomophthalic anhydride and 2,3-dimethoxybenzaldehyde in presence of DMAP/CH2Cl2 [8,9,10,11]. It has to be also mentioned that the target coumarin 3 was isolated in a high purity (>99%) and yield (92%) just by filtration at the end of the reaction, the latter suggesting the proposed reaction credible for the synthesis of a series of polyhydroxylated coumarin derivatives from readily available starting materials at a low cost.
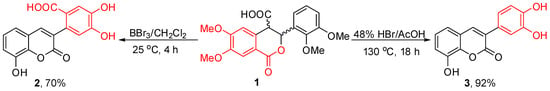
Scheme 1.
Synthesis of 3-(3,4-dihydroxyphenyl)-8-hydroxy-2H-chromen-2-one (3).
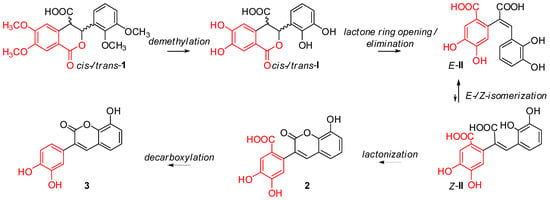
Scheme 2.
Proposed reaction mechanism for synthesis of 3-arylcoumarin 3.
3. Experimental Section
3.1. General Information
All chemicals used in this study were purchased from Sigma Aldrich (FOT, Sofia, Bulgaria). Melting points were determined on a Kofler microscope Boetius PHMK 0.5 (VEB Kombinat Nagema, Radebeul, Germany). The reactions were monitored by means of thin layer chromatography (TLC) on pre-coated polyesters sheets POLIGRAM® SIL G/UV254 (Merck, Darmstadt, Germany); spots were visualized with UV light. The IR spectra were acquired in Nujol on a Specord 75 (Analytik Jena, Jena, Germany) and are reported in reciprocal centimeters. NMR spectra were recorded on a Bruker Avance (250.13 MHz and 62.90 MHz for 1H and 13C, respectively,Bruker BioSpin GmbH, Rheinstetten, Germany) using DMSO-d6 as a solvent and TMS as an internal standard. The chemicals shifts (δ) are given in ppm relative to tetramethylsilane as an internal standard and coupling constants (J) values are reported in Hz. The exact mass of compound 3 was determined by HRMS analyses on DFS High Resolution GC/MS (Thermo, ACM2, Sofia, Bulgaria), after derivatization with diazomethane.
3.2. 3-(3,4-Dihydroxyphenyl)-8-hydroxy-2H-chromen-2-one
To a solution of cis-/trans-3-(2,3-dimethoxyphenyl)-6,7-dimethoxy-1-oxoisochroman-4-carboxylic acid (1.0 g, 2.6 mmol) (1) in acetic acid (4 mL), 48% HBr (5 mL) was added and the resulting mixture was stirred under reflux (130 °C) 18 h. At the end of the reaction (TLC), the reaction mixture was poured over grinded ice (50 g) and stirred for additional 30 min. The crystallized coumarin 3 was then filtered off, washed with deionized water and dried at 100 °C under reduced pressure. The obtained in this way coumarin 3 was recrystallized from acetic acid/water prior to spectral characterization (For NMR spectra see Supplementary materials).
Yield: 0.64 g (92%), greenish amorphous crystals, m.p. 278–280 °C. Lit. m.p. 255 °C (as monohydrate, Ref. [12]).
1H-NMR (250.13 MHz, DMSO-d6): δ = 6.81 (1H, d, 3JH,H = 8.3 Hz, =CH–), 7.02–7.10 (2H, m, =CH–), 7.09–7.21 (2H, m, =CH–), 7.24 (1H, d, 3JH,H = 2.2 Hz, =CH–), 8.04 (1H, s, =CH–) 9.17 (2H, brs, OH), 10.07 (1H, brs, OH).
13C-NMR (62.90 МHz, DMSO-d6): δ = 115.2 (=CH–), 115.9 (=CH–), 117.3 (=CH–), 118.2 (=CH–), 119.8 (=CH–), 120.6 (C), 124.3 (=CH–), 125.6 (C), 126.6 (C), 138.7 (=CH–), 141.2 (C), 144.1 (C), 144.7 (C), 146.1 (C), 159.6 (C).
IR: νО–Н = 3480–2400 cm−1, νC=O = 1700 cm−1.
HRMS (EI): m/z Calculated for C18H16O5: 312.09977. Found: 312.09812.
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3Supplementary File 4Acknowledgments
The financial support of the National Science Fund of Bulgaria at the Ministry of Education, Youth and Science (project DMU-03-10/2011) is greatly acknowledged by the authors. The authors are also thankful to Mrs. Silviya Stoykova for performing MS analyses.
Author Contributions
Ivan Svinyarov and Milen Bogdanov contributed equally to this work.
Conflicts of Interest
The authors declare no conflict of interest.
References and Notes
- Estévez-Braun, A.; González, A.G. Coumarins. Nat. Prod. Rep. 1997, 14, 465–475. [Google Scholar] [CrossRef] [PubMed]
- Mansfield, J.W. Antimicrobial compounds and resistance: The role of phytoalexins and phytoanticipins. In Mechanisms of Resistance to Plant Diseases; Slusarenko, A.J., Fraser, R.S.S., van Loon, L.C., Eds.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2000; Chapter 7; pp. 325–370. [Google Scholar]
- Gnonlonfin, G.J.B.; Sanni, A.; Brimer, L. Review scopoletin—Coumarin phytoalexin with medicinal properties. Crit. Rev. Plant Sci. 2012, 31, 47–56. [Google Scholar] [CrossRef]
- Nikhil, B.; Shikhra, B.; Anil, P.; Prakish, N.B. Diverse pharmacological activities of 3-substituted coumarins: A review. Int. Res. J. Pharm. 2012, 3, 24–29. [Google Scholar]
- Riveiro, M.E.; de Kimpe, N.; Moglioni, A.; Vázquez, R.; Monczor, F.; Shayo, C.; Davio, C. Coumarins: Old compounds with novel promising therapeutic perspectives. Curr. Med. Chem. 2010, 17, 1325–1338. [Google Scholar] [CrossRef] [PubMed]
- Fylaktakidou, K.; Hadjipavlou-Litina, D.; Litinas, K.; Nicolaides, D. Natural and synthetic coumarin derivatives with anti-inflammatory/antioxidant activities. Curr. Pharm. Des. 2004, 10, 3813–3833. [Google Scholar] [CrossRef] [PubMed]
- Hoult, J.R.; Payá, M. Pharmacological and biochemical actions of simple coumarins: Natural products with therapeutic potential. Gen. Pharmacol. 1996, 27, 713–722. [Google Scholar] [CrossRef]
- Miliovsky, M.; Svinyarov, I.; Mitrev, Y.; Evstatieva, Y.; Nikolova, D.; Chochkova, M.; Bogdanov, M. A novel one-pot synthesis and preliminary biological activity evaluation of cis-restricted polyhydroxy stilbenes incorporating protocatechuic acid and cinnamic acid fragments. Eur. J. Med. Chem. 2013, 66, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Miliovsky, M.; Svinyarov, I.; Prokopova, E.; Batovska, D.; Stoyanov, S.; Bogdanov, M.G. Synthesis and antioxidant activity of polyhydroxylated trans-restricted 2-arylcinnamic acids. Molecules 2015, 20, 2555–2575. [Google Scholar] [CrossRef] [PubMed]
- Svinyarov, I.; Bogdanov, M.G. One-pot synthesis and radical scavenging activity of novel polyhydroxylated 3-arylcoumarins. Eur. J. Med. Chem. 2014, 78, 198–206. [Google Scholar] [CrossRef] [PubMed]
- Bogdanov, M.; Palamareva, M. cis-/trans-Isochromanones. DMAP induced cycloaddition of homophthalic anhydride and aldehydes. Tetrahedron 2004, 60, 2525–2530. [Google Scholar] [CrossRef]
- Buu-Hoi, N.P.; Saint-Ruf, G.; Lobert, B. Oxygen heterocycles. Part XIV. Hydroxylated 3-aryl and 3-pyridylcoumarins. J. Chem. Soc. C 1969, 16, 2069–2070. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).