3-(3,4-Dihydroxyphenyl)-8-hydroxy-2H-chromen-2-one
Abstract
:1. Introduction
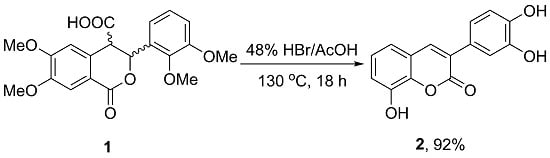
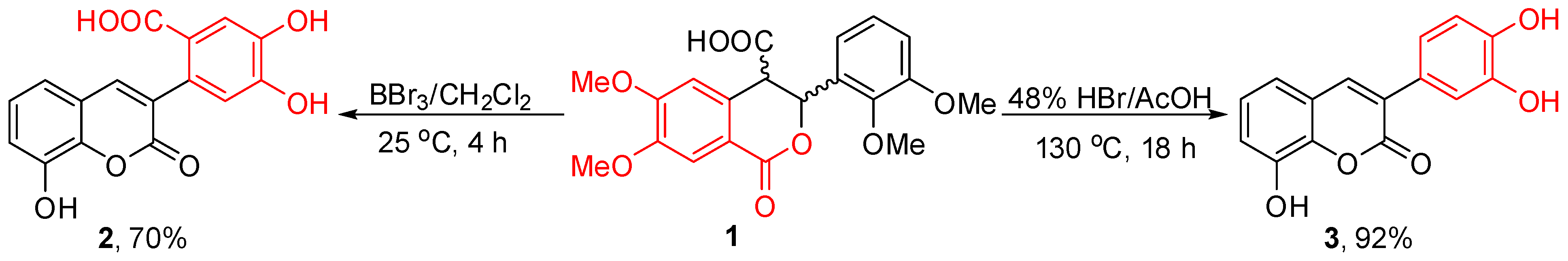
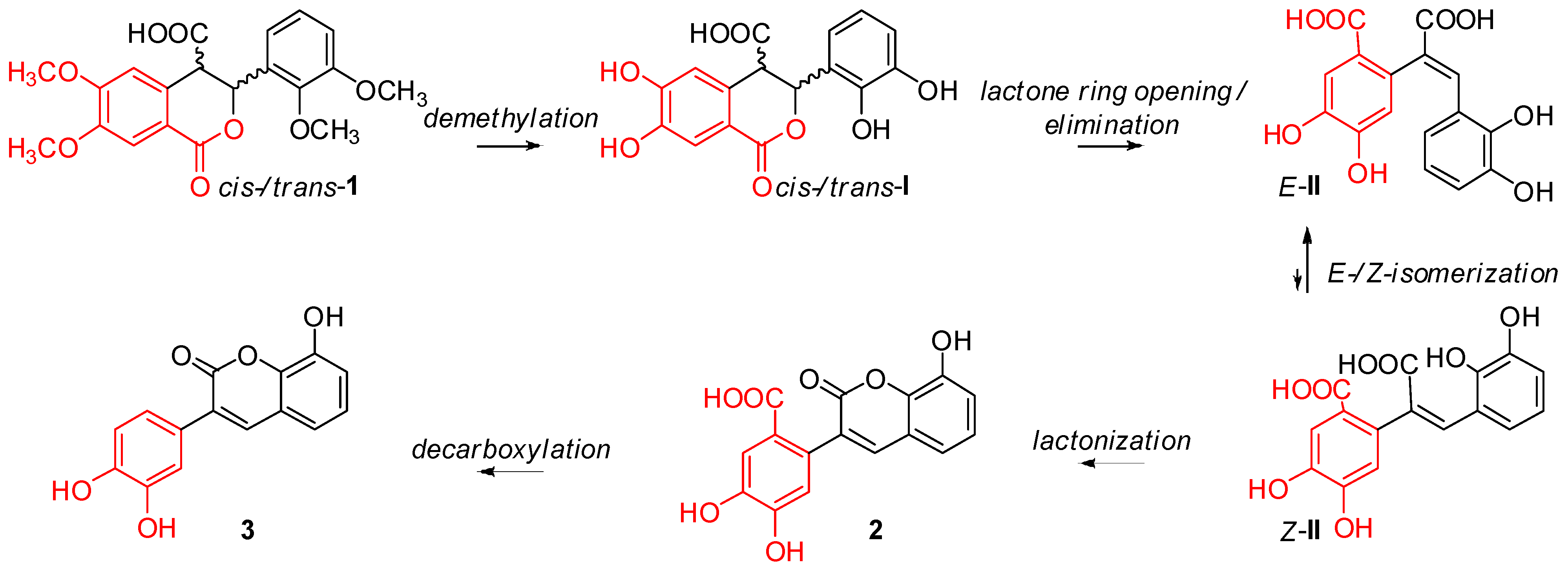
2. Results and Discussion
3. Experimental Section
3.1. General Information
3.2. 3-(3,4-Dihydroxyphenyl)-8-hydroxy-2H-chromen-2-one
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3Supplementary File 4Acknowledgments
Author Contributions
Conflicts of Interest
References and Notes
- Estévez-Braun, A.; González, A.G. Coumarins. Nat. Prod. Rep. 1997, 14, 465–475. [Google Scholar] [CrossRef] [PubMed]
- Mansfield, J.W. Antimicrobial compounds and resistance: The role of phytoalexins and phytoanticipins. In Mechanisms of Resistance to Plant Diseases; Slusarenko, A.J., Fraser, R.S.S., van Loon, L.C., Eds.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2000; Chapter 7; pp. 325–370. [Google Scholar]
- Gnonlonfin, G.J.B.; Sanni, A.; Brimer, L. Review scopoletin—Coumarin phytoalexin with medicinal properties. Crit. Rev. Plant Sci. 2012, 31, 47–56. [Google Scholar] [CrossRef]
- Nikhil, B.; Shikhra, B.; Anil, P.; Prakish, N.B. Diverse pharmacological activities of 3-substituted coumarins: A review. Int. Res. J. Pharm. 2012, 3, 24–29. [Google Scholar]
- Riveiro, M.E.; de Kimpe, N.; Moglioni, A.; Vázquez, R.; Monczor, F.; Shayo, C.; Davio, C. Coumarins: Old compounds with novel promising therapeutic perspectives. Curr. Med. Chem. 2010, 17, 1325–1338. [Google Scholar] [CrossRef] [PubMed]
- Fylaktakidou, K.; Hadjipavlou-Litina, D.; Litinas, K.; Nicolaides, D. Natural and synthetic coumarin derivatives with anti-inflammatory/antioxidant activities. Curr. Pharm. Des. 2004, 10, 3813–3833. [Google Scholar] [CrossRef] [PubMed]
- Hoult, J.R.; Payá, M. Pharmacological and biochemical actions of simple coumarins: Natural products with therapeutic potential. Gen. Pharmacol. 1996, 27, 713–722. [Google Scholar] [CrossRef]
- Miliovsky, M.; Svinyarov, I.; Mitrev, Y.; Evstatieva, Y.; Nikolova, D.; Chochkova, M.; Bogdanov, M. A novel one-pot synthesis and preliminary biological activity evaluation of cis-restricted polyhydroxy stilbenes incorporating protocatechuic acid and cinnamic acid fragments. Eur. J. Med. Chem. 2013, 66, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Miliovsky, M.; Svinyarov, I.; Prokopova, E.; Batovska, D.; Stoyanov, S.; Bogdanov, M.G. Synthesis and antioxidant activity of polyhydroxylated trans-restricted 2-arylcinnamic acids. Molecules 2015, 20, 2555–2575. [Google Scholar] [CrossRef] [PubMed]
- Svinyarov, I.; Bogdanov, M.G. One-pot synthesis and radical scavenging activity of novel polyhydroxylated 3-arylcoumarins. Eur. J. Med. Chem. 2014, 78, 198–206. [Google Scholar] [CrossRef] [PubMed]
- Bogdanov, M.; Palamareva, M. cis-/trans-Isochromanones. DMAP induced cycloaddition of homophthalic anhydride and aldehydes. Tetrahedron 2004, 60, 2525–2530. [Google Scholar] [CrossRef]
- Buu-Hoi, N.P.; Saint-Ruf, G.; Lobert, B. Oxygen heterocycles. Part XIV. Hydroxylated 3-aryl and 3-pyridylcoumarins. J. Chem. Soc. C 1969, 16, 2069–2070. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Svinyarov, I.; Bogdanov, M. 3-(3,4-Dihydroxyphenyl)-8-hydroxy-2H-chromen-2-one. Molbank 2015, 2015, M870. https://doi.org/10.3390/M870
Svinyarov I, Bogdanov M. 3-(3,4-Dihydroxyphenyl)-8-hydroxy-2H-chromen-2-one. Molbank. 2015; 2015(4):M870. https://doi.org/10.3390/M870
Chicago/Turabian StyleSvinyarov, Ivan, and Milen Bogdanov. 2015. "3-(3,4-Dihydroxyphenyl)-8-hydroxy-2H-chromen-2-one" Molbank 2015, no. 4: M870. https://doi.org/10.3390/M870
APA StyleSvinyarov, I., & Bogdanov, M. (2015). 3-(3,4-Dihydroxyphenyl)-8-hydroxy-2H-chromen-2-one. Molbank, 2015(4), M870. https://doi.org/10.3390/M870