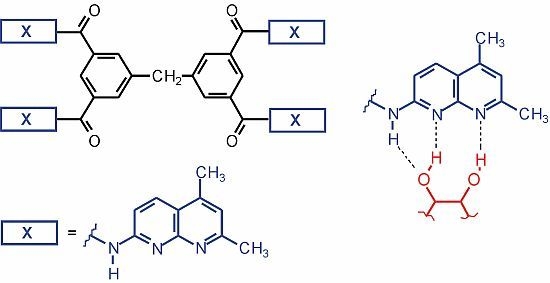
N,N',N",N"'-Tetrakis(5,7-dimethyl-1,8-naphthyridine-2-yl)-3,3',5,5'-diphenylmethanetetracarboxamide
Abstract
:Experimental
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3Supplementary File 4Acknowledgments
Author Contributions
Conflicts of Interest
References
- Koch, N.; Rosien, J.-R.; Mazik, M. Synthesis of compounds based on a dimesitylmethane scaffold and representative binding studies showing di- vs. monosaccharide preference. Tetrahedron 2014, 70, 8758–8767. [Google Scholar] [CrossRef]
- Mazik, M.; Buthe, A.C. Recognition properties of receptors based on dimesitylmethane-derived core: Di- vs. monosaccharide preference. Org. Biomol. Chem. 2009, 7, 2063–2071. [Google Scholar] [CrossRef] [PubMed]
- Mazik, M.; Koenig, A. Mimicking the binding motifs found in the crystal structures of protein-carbohydrate complexes: An aromatic analogue of serine or threonine side chain hydroxyl/main chain amide. Eur. J. Org. Chem. 2007, 2007, 3271–3276. [Google Scholar] [CrossRef]
- Mazik, M. Molecular recognition of carbohydrates by acyclic receptors employing noncovalent interactions. Chem. Soc. Rev. 2009, 38, 935–956. [Google Scholar] [CrossRef] [PubMed]
- Mazik, M. Recent developments in the molecular recognition of carbohydrates by artificial receptors. RSC Adv. 2012, 2, 2630–2642. [Google Scholar] [CrossRef]
- Mazik, M.; Sicking, W. Molecular recognition of carbohydrates by artificial receptors: Systematic studies towards recognition motifs for carbohydrates. Chem. Eur. J. 2001, 7, 664–670. [Google Scholar] [CrossRef]
- Mazik, M.; Cavga, H. An acyclic aminonaphthyridine-based receptor for carbohydrate recognition: Binding studies in competitive solvents. Eur. J. Org. Chem. 2007, 3633–3638. [Google Scholar] [CrossRef]
- Davis, A.P. Synthetic lectins. Org. Biomol. Chem. 2009, 7, 3629–3638. [Google Scholar] [CrossRef] [PubMed]
- Walker, D.B.; Joshi, G.; Davis, A.P. Progress in biomimetic carbohydrate recognition. Cell. Mol. Life Sci. 2009, 66, 3177–3191. [Google Scholar] [CrossRef] [PubMed]
- Le Blanc, J.R.; Sharp, D.B.; Murray, J.G. Di- and tetracarboxydiphenylmethanes and derivatives. J. Org. Chem. 1961, 26, 4731–4733. [Google Scholar] [CrossRef]
- Bernstein, J.; Stearns, B.; Shaw, E.; Lott, W.A. Derivatives of 2,6-Diaminopyridine. J. Am. Chem. Soc. 1947, 69, 1151–1158. [Google Scholar] [CrossRef] [PubMed]

© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schmidt, U.; Mazik, M. N,N',N",N"'-Tetrakis(5,7-dimethyl-1,8-naphthyridine-2-yl)-3,3',5,5'-diphenylmethanetetracarboxamide. Molbank 2015, 2015, M844. https://doi.org/10.3390/M844
Schmidt U, Mazik M. N,N',N",N"'-Tetrakis(5,7-dimethyl-1,8-naphthyridine-2-yl)-3,3',5,5'-diphenylmethanetetracarboxamide. Molbank. 2015; 2015(1):M844. https://doi.org/10.3390/M844
Chicago/Turabian StyleSchmidt, Ute, and Monika Mazik. 2015. "N,N',N",N"'-Tetrakis(5,7-dimethyl-1,8-naphthyridine-2-yl)-3,3',5,5'-diphenylmethanetetracarboxamide" Molbank 2015, no. 1: M844. https://doi.org/10.3390/M844
APA StyleSchmidt, U., & Mazik, M. (2015). N,N',N",N"'-Tetrakis(5,7-dimethyl-1,8-naphthyridine-2-yl)-3,3',5,5'-diphenylmethanetetracarboxamide. Molbank, 2015(1), M844. https://doi.org/10.3390/M844