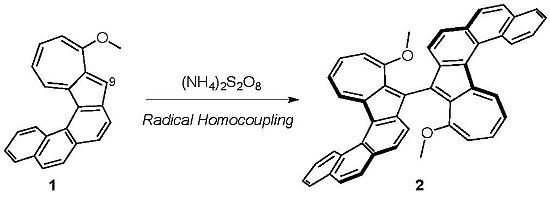
10,10'-Dimethoxy-9,9'-biazuleno[2,1-c]phenanthrene
Abstract
:Experimental Section
General Information
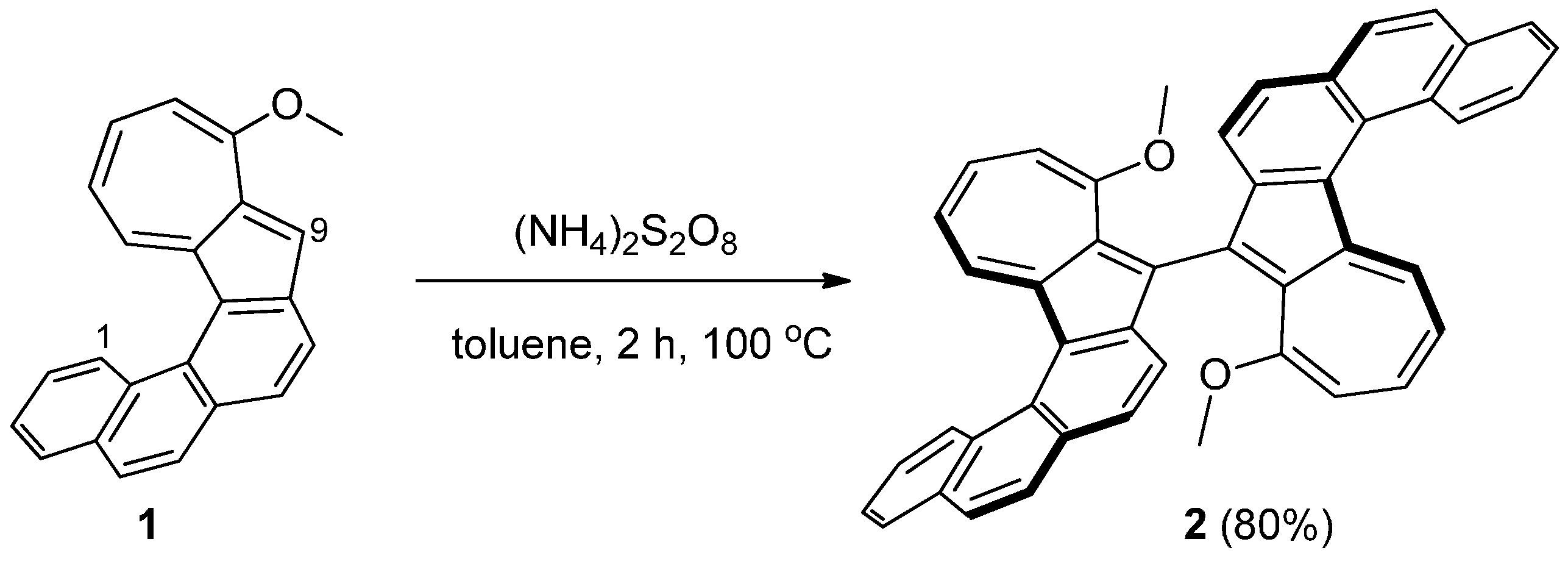
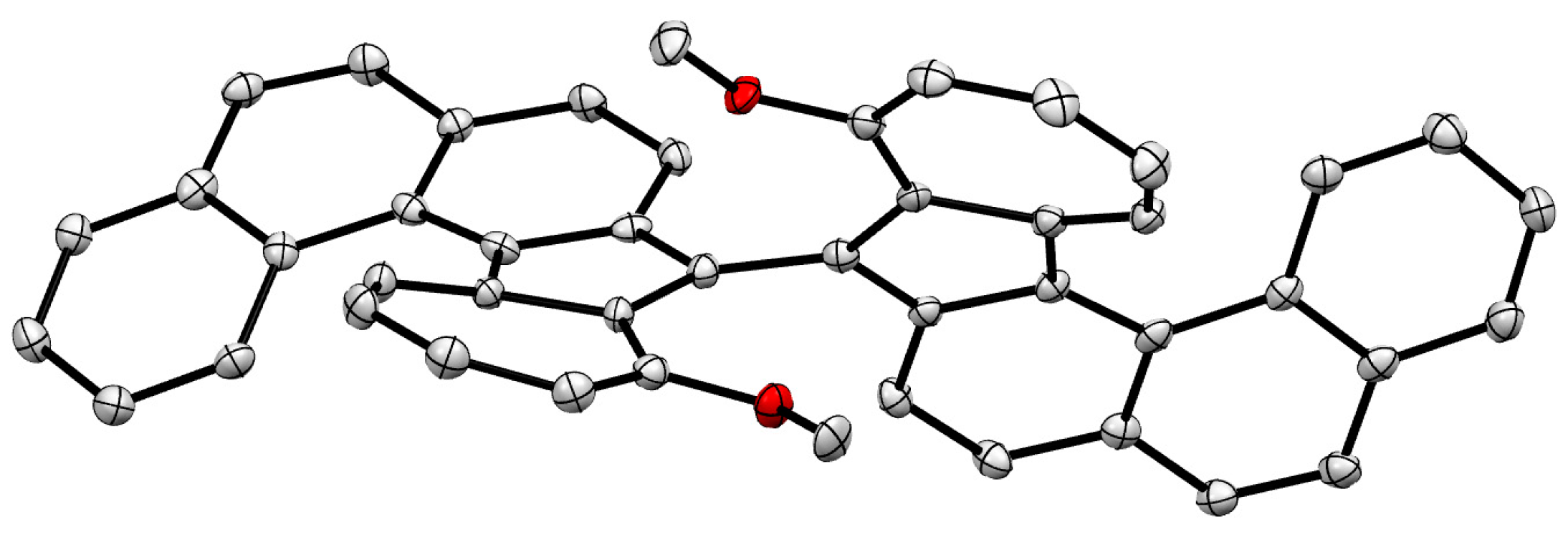
Synthesis of 10,10'-Dimethoxy-9,9'-biazuleno[2,1-c]phenanthrene (2)
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3Supplementary File 4Acknowledgments
Author Contributions
Conflicts of Interest
References and Notes
- Shoji, T.; Ito, S.; Toyota, K.; Yasunami, M.; Morita, N. The novel transition metal free synthesis of 1,1’-biazulene. Tetrahedron Lett. 2007, 48, 4999–5002. [Google Scholar] [CrossRef]
- Shoji, T.; Shimomura, E.; Inoue, Y.; Maruyama, M.; Yamamoto, A.; Fujimori, K.; Ito, S.; Yasunami, M.; Morita, N. Synthesis of novel thiophene-Fused 1,1'-biazulene derivative by the reaction of azuleno[1,2-b]thiophene with N-iodosuccinimide. Heterocycles 2013, 87, 303–306. [Google Scholar] [CrossRef]
- Bindl, J.; Seitz, P.; Seitz, U.; Salbeck, E.; Salbeck, J.; Daub, J. Elektronentransferreaktionen nichtalternierender chinoider und hydrochinoider verbindungen verhalten von 5,12-dimethoxynaphth[2,3-a]azulen bei der oxidation. Chem. Ber. 1987, 120, 1747–1756. [Google Scholar] [CrossRef]
- Yamamoto, K.; Okazumi, M.; Suemune, H.; Usui, K. Synthesis of [5]helicenes with a substituent exclusively on the interior side of the helix by metal-catalyzed cycloisomerization. Org. Lett. 2013, 15, 1806–1809. [Google Scholar] [CrossRef] [PubMed]
- Usui, K.; Tanoue, K.; Yamamoto, K.; Shimizu, T.; Suemune, H. Synthesis of substituted azulenes via Pt(II)-catalyzed ring-expanding cycloisomerization. Org. Lett. 2014, 16, 4662–4665. [Google Scholar] [CrossRef] [PubMed]
- Other reagents, such as N-iodosuccinimide (NIS) [2] and FeCl3 [3], were tested in this reaction, but none was superior to APS.
- The Cambridge Crystallographic Data Centre. CCDC 1041294 (2) Contain the Supplementary Crystallographic Data for this Paper. Available online: www.ccdc.cam.ac.uk/data_request/cif (accessed on 29 December 2014).
- Kurihara, T.; Suzuki, T.; Wakabayashi, H.; Ishikawa, S.; Shindo, K.; Shimada, Y.; Chiba, H.; Miyashi, T.; Yasunami, M.; Nozoe, T. Electronic structures and oxidation potentials of some azulene derivatives. Bull. Chem. Soc. Jpn. 1996, 69, 2003–2008. [Google Scholar] [CrossRef]
- Suzuki, T.; Saito, M.; Kawai, H.; Fujiwara, K.; Tsuji, T. Preparation, properties, and X-ray structures of 5,5'-bi(8-aminoquinoxalyl)s: Novel Wurster-type electron donors with a heterobiaryl skeleton. Tetrahedron Lett. 2004, 45, 329–333. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yamamoto, K.; Nakamae, R.; Suemune, H.; Usui, K. 10,10'-Dimethoxy-9,9'-biazuleno[2,1-c]phenanthrene. Molbank 2015, 2015, M843. https://doi.org/10.3390/M843
Yamamoto K, Nakamae R, Suemune H, Usui K. 10,10'-Dimethoxy-9,9'-biazuleno[2,1-c]phenanthrene. Molbank. 2015; 2015(1):M843. https://doi.org/10.3390/M843
Chicago/Turabian StyleYamamoto, Kosuke, Ryota Nakamae, Hiroshi Suemune, and Kazuteru Usui. 2015. "10,10'-Dimethoxy-9,9'-biazuleno[2,1-c]phenanthrene" Molbank 2015, no. 1: M843. https://doi.org/10.3390/M843
APA StyleYamamoto, K., Nakamae, R., Suemune, H., & Usui, K. (2015). 10,10'-Dimethoxy-9,9'-biazuleno[2,1-c]phenanthrene. Molbank, 2015(1), M843. https://doi.org/10.3390/M843