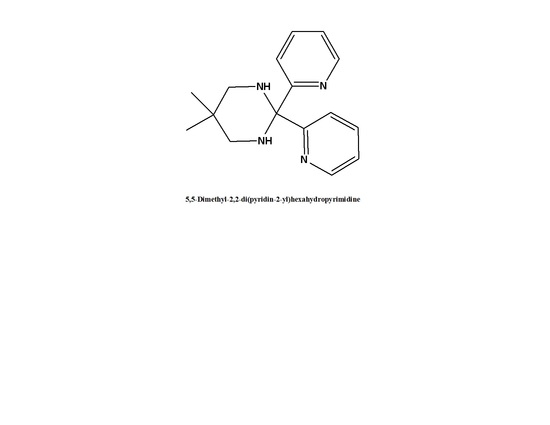
5,5-Dimethyl-2,2-di(pyridin-2-yl)hexahydropyrimidine
Abstract
:Introduction
Result and Discussion
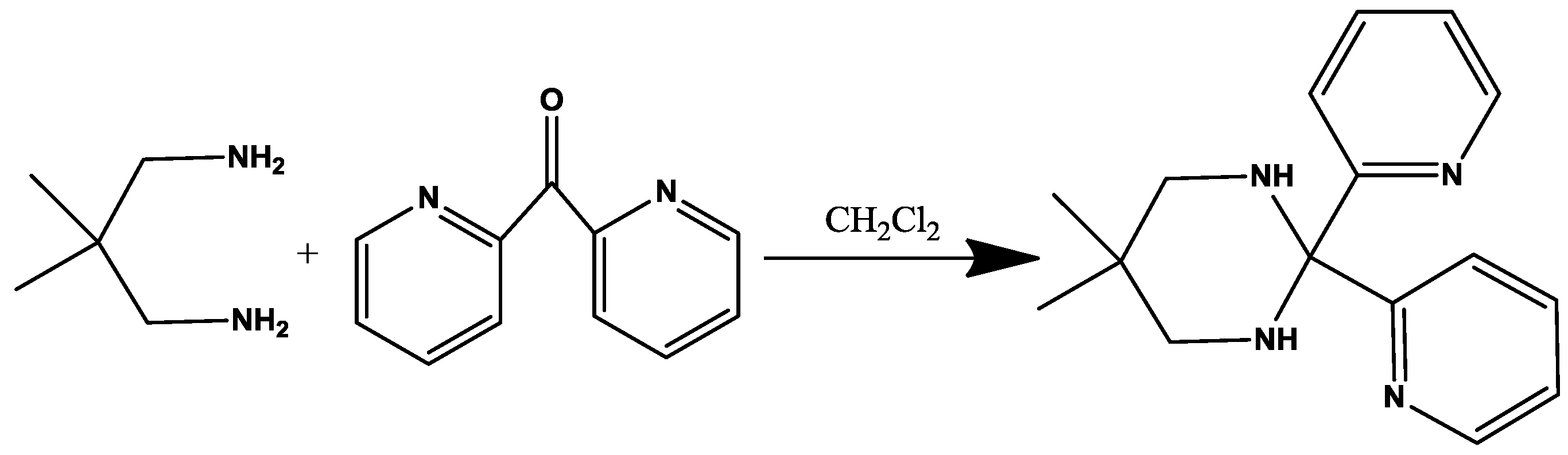
Experimental Section
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3Acknowledgments
Author Contributions
Conflicts of Interest
References
- Perillo, I.A.; Garcia, M.B.; Bisceglia, J.A.; Orelli, L.R. N-Arylhexahydropyrimidines. Part 1. Synthesis and 1H NMR Characterization of 1,3-Di and 1,2,3-Trisubstituted Derivatives. J. Heterocycl. Chem. 2002, 39, 655–661. [Google Scholar] [CrossRef]
- Drandarov, K.; Guggisberg, A.; Hesse, M. Macrocyclic Spermine Alkaloids from Verbascum: The (E/Z)-Isomeric Pairs (−)-(S)-Verbasitrine/(−)-(S)-Isoverbasitrine and (+)-(S)-Verbametrine/(+)-(S)-Isoverbametrine: Isolation, Structure Elucidation, and Synthesis. Helv. Chim. Acta 1999, 82, 229–237. [Google Scholar] [CrossRef]
- Hrvath, D.J. A Virtual Screening Approach Applied to the Search for Trypanothione Reductase Inhibitors. J. Med. Chem. 1999, 40, 2412–2423. [Google Scholar] [CrossRef] [PubMed]
- Mayr, M.; Wurst, K.; Ongania, K.-H.; Buchmester, M.R. 1,3-Dialkyl- and 1,3-Diaryl-3,4,5,6-tetrahydropyrimidin-2-ylidene Rhodium(i) and Palladium(II) Complexes: Synthesis, Structure, and Reactivity. Chem.-Eur. J. 2004, 10, 1256–1266. [Google Scholar] [CrossRef] [PubMed]
- Mayr, M.; Buchmeister, M.R. Rapid Screening of New Polymer-Supported Palladium(II) Bis(3,4,5,6-tetrahydropyrimidin-2-ylidenes. Macromol. Rapid Commun. 2004, 25, 231–236. [Google Scholar] [CrossRef]
- Katritzky, A.R.; Singh, S.K.; He, H.-Y. Novel Syntheses of Hexahydropyrimidines and Tetrahydroquinazolines. J. Org. Chem. 2002, 67, 3115–3117. [Google Scholar] [CrossRef] [PubMed]
- Ren, C.-X.; Ye, B.-H.; Zhu, H.-L.; Shi, J.-X.; Chen, X.-M. Syntheses, Structures and Photo luminescentProperties of Silver(I) Complexes with in situ Generated Hexahydropyrimidine Derivatives. Inorg. Chim. Acta 2004, 357, 443–450. [Google Scholar] [CrossRef]
- Schmidt, M.; Wiedemann, D.; Grohmann, A. First-row Transition Metal Complexes of a Novel Pentadentate Amine/Imine Ligand Containing a Hexahydropyrimidine Core. Inorg. Chim. Acta 2011, 374, 514–520. [Google Scholar] [CrossRef]
- Azam, M.; Ismail Warad, I.; Al-Resayes, S.; Alzaqri, N.; Khan, M.; Pallepogu, R.; Dwivedi, S.; Musarrat, J.; Shakir, M. Synthesis and structural characterization of Pd(II) complexes derived from perimidine ligand and their in vitro antimicrobial studies. J. Mol. Struct. 2013, 1047, 48–54. [Google Scholar] [CrossRef]
- Warad, I.; Alruwaili, A.; Al-Resayes, S.; Choudhary, M.I.; Yousuf, Y. 5,5-Dimethyl-2,2-bis(pyridin-2-yl)-1,3-diazinane. Acta Crystallogr. Sect. E: Struct. Rep. Online 2012, 68, o1786. [Google Scholar] [CrossRef] [PubMed]
- Haddad, S.F.; Warad, I.; Jodeh, S.; Ben Hadda, T. 2,2-Bis(pyridin-2-yl)-1,3-diazinane. Acta Crystallogr. Sect. E: Struct. Rep. Online 2013, 69, o569. [Google Scholar] [CrossRef] [PubMed]



© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abu-Obaid, A.; Asadi, A.I.; Alruwaili, A.; Atieh, H.; Khlaif, S.; Hadda, T.B.; Radi, S.; Hammouti, B.; Warad, I. 5,5-Dimethyl-2,2-di(pyridin-2-yl)hexahydropyrimidine. Molbank 2015, 2015, M838. https://doi.org/10.3390/M838
Abu-Obaid A, Asadi AI, Alruwaili A, Atieh H, Khlaif S, Hadda TB, Radi S, Hammouti B, Warad I. 5,5-Dimethyl-2,2-di(pyridin-2-yl)hexahydropyrimidine. Molbank. 2015; 2015(1):M838. https://doi.org/10.3390/M838
Chicago/Turabian StyleAbu-Obaid, Ahmad., Ahmad I. Asadi, Afaf. Alruwaili, Heba. Atieh, Shoqour Khlaif, Taibi Ben Hadda, Smaail Radi, Belkheir Hammouti, and Ismail Warad. 2015. "5,5-Dimethyl-2,2-di(pyridin-2-yl)hexahydropyrimidine" Molbank 2015, no. 1: M838. https://doi.org/10.3390/M838
APA StyleAbu-Obaid, A., Asadi, A. I., Alruwaili, A., Atieh, H., Khlaif, S., Hadda, T. B., Radi, S., Hammouti, B., & Warad, I. (2015). 5,5-Dimethyl-2,2-di(pyridin-2-yl)hexahydropyrimidine. Molbank, 2015(1), M838. https://doi.org/10.3390/M838