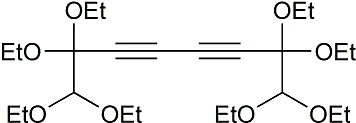
1,1,2,2,7,7,8,8-Octaethoxyocta-3,5-diyne
Abstract
:Experimental Section
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3Supplementary File 4Acknowledgements
Author Contributions
Conflicts of Interest
References
- Raphael, R.A. Acetylenic Compounds in Organic Synthesis; Butterworths Scientific Publications: London, UK, 1955. [Google Scholar]
- Viehe, H.G. (Ed.) Chemistry of Acetylenes; Marcel Dekker: New York, USA, 1969.
- Niedballa, U. Di- und Polyine. In Methoden der Organischen Chemie (Houben-Weyl)Band V/2a, 4th ed.; Jäger, V., Murray, M., Niedballa, U., Viehe, H.G., Eds.; Georg Thieme Verlag: Stuttgart, Germany, 1977. [Google Scholar]
- Larock, R.C. Comprehensive Organic Transformations: A Guide to Functional Group Preparations; VCH Publishers: New York, USA, 1989. [Google Scholar]
- Siemsen, P.; Livingston, R.C.; Diederich, F. Acetylenic Coupling: A Powerful Tool in Molecular Construction. Angew. Chem. Int. Ed. 2000, 39, 2632–2657. [Google Scholar] [CrossRef]
- Shi, W.; Lei, A. 1,3-Diyne Chemistry: synthesis and derivations. Tetrahedron Lett. 2014, 55, 2763–2772. [Google Scholar] [CrossRef]
- Evano, G.; Blanchard, N.; Toumi, M. Copper-Mediated Coupling Reactions and Their Applications in Natural Products and Designed Biomolecules Synthesis. Chem. Rev. 2008, 108, 3054–3131. [Google Scholar] [CrossRef] [PubMed]
- Sydnes, L.K.; Holmelid, B.; Kvernenes, O.H.; Sandberg, M.; Hodne, M.; Bakstad, E. Synthesis and some chemical properties of 3,3,4,4-tetraethoxybut-1-yne. Tetrahedron 2007, 63, 4144–4148. [Google Scholar] [CrossRef]
- Sydnes, L.K.; Valdersnes, S. Recent advances in the synthesis of carbohydrate analogues. Pure Appl. Chem. 2007, 79, 2137–2142. [Google Scholar] [CrossRef]
- Sydnes, L.K.; Holmelid, B.; Kvernenes, O.H.; Valdersnes, S.; Hodne, M.; Boman, K. Stereospecific synthesis of allylic and homoallylic alcohols from functionalized propargylic alcohols. ARKIVOC 2008, xiv, 242–268. [Google Scholar]
- Sydnes, L.K.; Holmelid, B.; Sengee, M.; Hanstein, M. New regiospecific synthesis of tri- and tetrasubstituted furans. J. Org. Chem. 2009, 74, 3430–3443. [Google Scholar] [CrossRef] [PubMed]
- Sydnes, L.K.; Isanov, R.; Sengee, M.; Livi, F. Regiospecific Synthesis of Tetra-Substituted Furans. Synth. Commun. 2013, 43, 2898–2905. [Google Scholar] [CrossRef]
- Erdenebileg, U.; Høstmark, I.; Polden, K.; Sydnes, L.K. Synthesis and reactivity of 4-amino-sustituted furfurals. J. Org. Chem. 2014, 79, 1213–1221. [Google Scholar] [CrossRef] [PubMed]
- Farooq, T.; Haug, B.E.; Sydnes, L.K.; Tӧrnroos, K.W. 1,3-Dipolar cycloaddition of benzyl azide to two highly functionalized alkynes. Monatsh. Chem. 2012, 143, 505–512. [Google Scholar] [CrossRef] [Green Version]
- Sydnes, L.K.; Kvernenes, O.H.; Valdersnes, S. From 3,3,4,4-tetraethoxybutyne to carbohydrate mimics. Pure Appl. Chem. 2005, 77, 119–130. [Google Scholar] [CrossRef]
- Valdersnes, S.; Sydnes, L.K. Preparation of 2-ethoxy-3-hydroxy-4-(perfluoroalkyl)tetrahydropyran derivatives from substituted 4-ethoxybut-3-en-1-ols. Eur. J. Org. Chem. 2009, 2009, 5816–5831. [Google Scholar] [CrossRef]
- Valdersnes, S.; Apeland, I.; Flemmen, G.; Sydnes, L.K. Toward the synthesis of modified carbohydrates by conjugate addition of propane-1,3-dithiol to α,β-unsaturated ketones. Helv. Chim. Acta 2012, 95, 2099–2122. [Google Scholar] [CrossRef]
- Sengee, M.; Sydnes, L.K. Michael Addition of Various Nitrogen and Oxygen Nucleophiles to 1,1-Diethoxybut-3-yn-2-one. Synthesis 2011, 3899–3907. [Google Scholar]
- Isanov, R.; Holmelid, B.; Törnroos, K.W.; Sydnes, L.K. Synthesis of (E)-1,1-diethoxy-3-(3-hydroxy-3-arylfuro[2,3-b]quinoxalin-2(3H)-ylidene)propan-2-ones via acid-catalyzed, stereoselective 5-Exo-Dig cyclization. J. Heterocycl. Chem. 2014. [Google Scholar] [CrossRef]
- Nes, I.; Sydnes, L.K. Formation of N-heterocycles from 1,1-diethoxy-5-hydroxyalk-3-yn-2-ones. Synthesis 2015, 47, 89–94. [Google Scholar]
- Leiren, M.K.; Valdersnes, S.; Sydnes, L.K. Selective transformations of a diprotected 2-oxo-butanedial. Helv. Chim. Acta 2013, 96, 1841–1850. [Google Scholar] [CrossRef]
- Holmelid, B. From Trihalocyclopropanes to Carbohydrate Analogues via Functionalized Alkynes. Ph.D. Thesis, University of Bergen, Norway, 2009. [Google Scholar]
- Sonogashira, K.; Tohda, Y.; Hagihara, N. A convenient synthesis of acetylenes: Catalytic substitution of acetylenic hydrogen with bromoalkenes, iodoarenes and bromopyridines. Tetrahedron Lett. 1975, 4467–4470. [Google Scholar] [CrossRef]
- Glaser, C. Beiträge zur Kenntniss des Acetenylbenzols. Ber. Dtsch. Chem. Ges. 1869, 2, 422–424. [Google Scholar] [CrossRef]
- Glaser, C. Untersuchungen über einige Derivate der Zimmtsaüre. Ann. Chem. Pharm. 1870, 154, 137–171. [Google Scholar] [CrossRef]
- Eglinton, G.; Galbraith, A.R. Cyclic diynes. Chem. Ind. (London) 1956, 737–738. [Google Scholar]
- Ebert, G.W.; Rieke, R.D. Preparation of Aryl, Alkynyl, and Vinyl Organocopper Compounds by the Oxidative Addition of Zerovalent Copper to Carbon-Halogen Bonds. J. Org. Chem. 1988, 53, 4482–4488. [Google Scholar] [CrossRef]
- Elangovan, A.; Wang, Y.-H.; Ho, T.-I. Sonogashira coupling reaction with diminished homo-coupling. Org. Lett. 2003, 5, 1841–1844. [Google Scholar] [CrossRef] [PubMed]
- Gelman, D.; Buchwald, S.L. Efficient palladium-catalyzed coupling of aryl chlorides and tosylates with terminal alkynes: Use of a copper cocatalyst inhibits the reaction. Angew. Chem. Int. Ed. 2003, 42, 5993–5996. [Google Scholar] [CrossRef] [PubMed]
- Beaupérin, M.; Job, A.; Cattey, H.; Royer, S.; Meunier, P.; Hierso, J.-C. Copper(I) iodide polyphosphine adducts at low loading for Sonogashira alkynylation of demanding halide substrates: Ligand exchange study between copper and palladium. Organometallics 2010, 29, 2815–2822. [Google Scholar] [CrossRef]

© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Holmelid, B.; Sydnes, L.K. 1,1,2,2,7,7,8,8-Octaethoxyocta-3,5-diyne. Molbank 2015, 2015, M840. https://doi.org/10.3390/M840
Holmelid B, Sydnes LK. 1,1,2,2,7,7,8,8-Octaethoxyocta-3,5-diyne. Molbank. 2015; 2015(1):M840. https://doi.org/10.3390/M840
Chicago/Turabian StyleHolmelid, Bjarte, and Leiv K. Sydnes. 2015. "1,1,2,2,7,7,8,8-Octaethoxyocta-3,5-diyne" Molbank 2015, no. 1: M840. https://doi.org/10.3390/M840
APA StyleHolmelid, B., & Sydnes, L. K. (2015). 1,1,2,2,7,7,8,8-Octaethoxyocta-3,5-diyne. Molbank, 2015(1), M840. https://doi.org/10.3390/M840