Benzyl {2-[(2-(1H-Benzo[d][1,2,3]triazol-1-yl)-2-oxoethyl)amino]-2-oxoethyl} carbamate †
Abstract
:Results and Discussion
Experimental Section
General Chemical Procedure
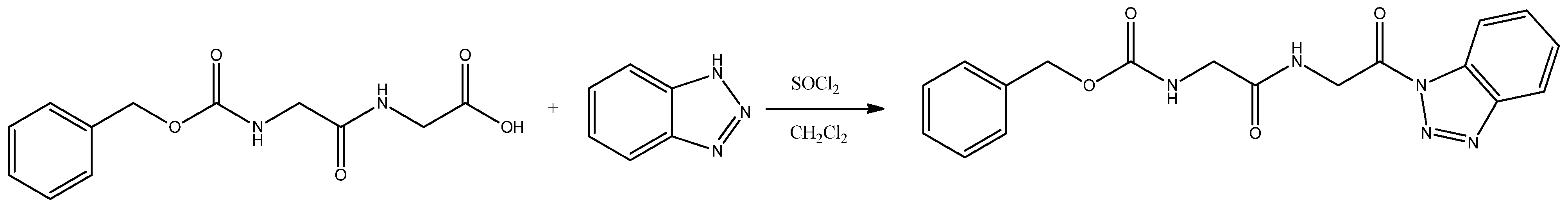
Synthesis of the Benzyl {2-[(2-(1H-Benzo[d][1,2,3]triazol-1-yl)-2-oxoethyl)amino]-2-oxoethyl}carbamate
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3Supplementary File 4Supplementary File 5Acknowledgments
Author Contributions
Conflicts of Interest
References
- Panda, S.S.; Hall, C.D.; Scriven, E.; Katritzky, A.R. Aminoacyl benzotriazoles: versatile reagents for the preparation of peptides and their mimetics and conjugates. Aldrichim. Acta 2013, 46, 43–55. [Google Scholar]
- Lucchese, G.; Stufano, A.; Trost, B.; Kusalik, A.; Kanduc, D. Peptidology: short amino acid modules in cell biology and immunology. Amino Acids 2007, 33, 703–707. [Google Scholar] [CrossRef] [PubMed]
- El Khatib, M.; Jauregui, L.; Tala, S.R.; Khelashvili, L.; Katritzky, A.R. Solution-phase synthesis of chiral O-acyl isodipeptides. Med. Chem. Commun. 2011, 2, 1087–1092. [Google Scholar] [CrossRef]
- Glunz, P.W.; Hintermann, S.; Williams, L.J.; Schwarz, J.B.; Kuduk, S.D.; Kudryashov, V.; Lloyd, K.O.; Danishefsky, S.J. Design and synthesis of Le(y)-bearing glycopeptides that mimic cell surface Le(y) mucin glycoprotein architecture. J. Am. Chem. Soc. 2000, 122, 7273–7279. [Google Scholar] [CrossRef]
- Katritzky, A.R.; Angrish, P.; Todadze, E. Chiral acylation with N-(protected α-aminoacyl)benzotriazoles for advantageous syntheses of peptides and peptide conjugates. Synlett 2009, 2009, 2392–2411. [Google Scholar] [CrossRef]
- Panda, S.S.; Ibrahim, M.A.; Küçükbay, H.; Meyers, M.J.; Sverdrup, F.M.; El-Feky, S.A.; Katritzky, A.R. Synthesis and antimalarial bioassay of quinine peptide conjugates. Chem. Biol. Drug Res. 2013, 82, 361–366. [Google Scholar] [CrossRef] [PubMed]
- Salam, S.M.A.; Kagawa, K.; Matsubara, T.; Kawashiro, K. Protease-catalyzed dipeptide synthesis from N-protected amino acid carbamoylmethyl esters and free amino acids in frozen aqueus solutions. Enzyme Microb. Technol. 2008, 43, 537–543. [Google Scholar] [CrossRef]
- Katritzky, A.R.; Vincek, A.S.; Suzuki, K. Microwave-assisted synthesis of peptidyl phosphorus ylides. ARKIVOC 2005, 5, 116–126. [Google Scholar]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Küçükbay, H.; Buğday, N. Benzyl {2-[(2-(1H-Benzo[d][1,2,3]triazol-1-yl)-2-oxoethyl)amino]-2-oxoethyl} carbamate. Molbank 2014, 2014, M828. https://doi.org/10.3390/M828
Küçükbay H, Buğday N. Benzyl {2-[(2-(1H-Benzo[d][1,2,3]triazol-1-yl)-2-oxoethyl)amino]-2-oxoethyl} carbamate. Molbank. 2014; 2014(3):M828. https://doi.org/10.3390/M828
Chicago/Turabian StyleKüçükbay, Hasan, and Nesrin Buğday. 2014. "Benzyl {2-[(2-(1H-Benzo[d][1,2,3]triazol-1-yl)-2-oxoethyl)amino]-2-oxoethyl} carbamate" Molbank 2014, no. 3: M828. https://doi.org/10.3390/M828
APA StyleKüçükbay, H., & Buğday, N. (2014). Benzyl {2-[(2-(1H-Benzo[d][1,2,3]triazol-1-yl)-2-oxoethyl)amino]-2-oxoethyl} carbamate. Molbank, 2014(3), M828. https://doi.org/10.3390/M828



