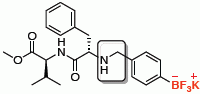
Potassium {4-[(3S,6S,9S)-3,6-dibenzyl-9-isopropyl-4,7,10-trioxo-11–oxa-2,5,8-triazadodecyl]phenyl}trifluoroborate
Abstract
:Result and Discussion
Experimental
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3Supplementary File 4Acknowledgments
Author Contributions
Conflicts of Interest
References
- Pivazyan, A.D.; Matteson, D.S.; Fabry-Asztalos, L.; Singh, R.P.; Lin, P.; Blair, W.; Guo, K.; Robinson, B.; Prusoff, W.H. Inhibition of HIV-1 protease by a boron-modified polypeptide. Biochem. Pharm. 2000, 60, 927–936. [Google Scholar] [CrossRef]
- Kim, N.N.; Cox, J.D.; Baggio, R.F.; Emig, F.A.; Mistry, S.K.; Harper, S.L.; Speicher, D.W.; Morris, S.M., Jr.; Ash, D.E.; Traish, A.; et al. Human Arginase II: Crystal Structure and Physiological Role in Male and Female Sexual Arousal. Biochemistry 2003, 42, 8445–8451. [Google Scholar]
- Priestley, E.S.; Decicco, C.P. 1-Aminocyclopropaneboronic Acid: Synthesis and Incorporation into an Inhibitor of Hepatitis C Virus NS3 Protease. Org. Lett. 2000, 2, 3095–3097. [Google Scholar] [PubMed]
- Priestley, E.S.; de Lucca, I.; Ghavimi, B.; Erickson-Viitanen, S.; Decicco, C.P. P1 Phenethyl peptide boronic acid inhibitors of HCV NS3 protease. Bioorg. Med. Chem. Lett. 2002, 12, 3199–3202. [Google Scholar] [CrossRef]
- Zhang, Y.-K.; Plattner, J.J.; Freund, Y.R.; Easom, E.E.; Zhou, Y.; Ye, L.; Zhou, H.; Waterson, D.; Gamo, F.-J.; Sanz, L.M.; et al. Benzoxaborole antimalarialagents.Part 2: Discovery of fluoro-substituted 7-(2-carboxyethyl)-1,3-dihydro-1-hydroxy-2,1-benzoxaboroles. Bioorg. Med. Chem. Lett. 2012, 22, 1299–1037. [Google Scholar] [CrossRef] [PubMed]
- Qiao, Z.; Wang, Q.; Zhang, F.; Wang, Z.; Bowling, T.; Nare, B.; Jacobs, R.T.; Zhang, J.; Ding, D.; Liu, Y.; et al. Chalcone–Benzoxaborole Hybrid Molecules as Potent Antitrypanosomal Agents. J. Med. Chem. 2012, 55, 3553–3557. [Google Scholar] [PubMed]
- Jabbour, A.; Steinberg, D.; Dembitsky, V.M.; Moussaieff, A.; Zaks, B.; Srebnik, M.J. Synthesis and Evaluation of Oxazaborolidines for Antibacterial Activity againstStreptococcus mutans. J. Med. Chem. 2004, 47, 2409–2410. [Google Scholar] [CrossRef] [PubMed]
- Jabbour, A.; Srebnik, M.; Zaks, B.; Dembitsky, V.; Steinberg, D. Evaluation of oxazaborolidine activity on Streptococcus mutansbiofilm formation. Int. J. Antimicrob. Agents. 2005, 26, 491–496. [Google Scholar] [CrossRef] [PubMed]
- Tsavalos, M.; Nicholson, B.C.; Spotswood, T.M. 19F nuclear magnetic resonance studies of the interaction of inhibitors with chymotrypsin. Ring-fluorinated derivatives of N-trifluoroacetylphenylalanine. Aust. J. Chem. 1978, 31, 2179–2186. [Google Scholar] [CrossRef]
- Smoum, R.; Rubinstein, A.; Srebnik, M. Noncovalent inhibition of the serine proteases, α-chymotrypsin and trypsin by trifluoro(organo)borates. Org. Biomol. Chem. 2005, 3, 941–944. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, R.A.; Savegnago, L.; Jesse, C.R.; Menezes, P.H.; Molander, G.A.; Nogueira, C.W. Toxicological Investigation and Antinociceptive Property of Potassium Thiophene-3-Trifluoroborate. Basic Clin. Pharmacol. Toxicol. 2009, 104, 448–454. [Google Scholar] [CrossRef] [PubMed]
- Pan, P-S.; McGuire, K.L.; McAlpine, S.R. Identification of Sansalvamide A analog potent against pancreatic cancer cell lines. Bioorg. Med. Chem. Lett. 2007, 17, 5072–5077. [Google Scholar]
- Burkhardt, E.R.; Coleridge, B.M. Reductive amination with 5-ethyl-2-methylpyridine borane. Tetrahedron Lett. 2008, 49, 5152–5155. [Google Scholar] [CrossRef]

© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Tsai, C.-H.; Lin, C.-H.; Hsieh, C.-T.; Cai, C.-C.; Lin, T.-J.; Liu, P.-Y.; Lin, M.-H.; Wu, M.-J.; Fu, C.-C.; Wu, Y.-C.; et al. Potassium {4-[(3S,6S,9S)-3,6-dibenzyl-9-isopropyl-4,7,10-trioxo-11–oxa-2,5,8-triazadodecyl]phenyl}trifluoroborate. Molbank 2014, 2014, M827. https://doi.org/10.3390/M827
Tsai C-H, Lin C-H, Hsieh C-T, Cai C-C, Lin T-J, Liu P-Y, Lin M-H, Wu M-J, Fu C-C, Wu Y-C, et al. Potassium {4-[(3S,6S,9S)-3,6-dibenzyl-9-isopropyl-4,7,10-trioxo-11–oxa-2,5,8-triazadodecyl]phenyl}trifluoroborate. Molbank. 2014; 2014(2):M827. https://doi.org/10.3390/M827
Chicago/Turabian StyleTsai, Chia-Hua, Chia-Hung Lin, Ching-Tien Hsieh, Chih-Cheng Cai, Ting-Ju Lin, Pin-Yi Liu, Meng-Hsuan Lin, Meng-Ju Wu, Chia-Chieh Fu, Yang-Chang Wu, and et al. 2014. "Potassium {4-[(3S,6S,9S)-3,6-dibenzyl-9-isopropyl-4,7,10-trioxo-11–oxa-2,5,8-triazadodecyl]phenyl}trifluoroborate" Molbank 2014, no. 2: M827. https://doi.org/10.3390/M827
APA StyleTsai, C.-H., Lin, C.-H., Hsieh, C.-T., Cai, C.-C., Lin, T.-J., Liu, P.-Y., Lin, M.-H., Wu, M.-J., Fu, C.-C., Wu, Y.-C., Chang, F.-R., & Pan, P.-S. (2014). Potassium {4-[(3S,6S,9S)-3,6-dibenzyl-9-isopropyl-4,7,10-trioxo-11–oxa-2,5,8-triazadodecyl]phenyl}trifluoroborate. Molbank, 2014(2), M827. https://doi.org/10.3390/M827