Abstract
Suzuki coupling reaction of 5-fluoro-6-methylpyridin-2-ylboronic acid (1) with 4-chloro-2-iodo-1-(trifluoromethyl)benzene (2) in the presence of dikis and K2CO3 produce C-C coupled new title compound 6-(5-chloro-2-(trifluoromethyl)phenyl)-3-fluoro-2-methylpyridine (3). The structure of the newly synthesized compound has been confirmed by IR, 1H-NMR, 13C-NMR, LC-MS and CHN analysis.
The fluorine atom plays a very important role in the chemical and biological field [1]. After nitrogen, fluorine occupies the position of the second most important hetero atom in life science research. Overall 10%–15% of newly synthesised pharmaceutical drugs and 30%–40% of newly registered agrochemicals contain one or more fluorine atom.[2] Generally, halogenated heterocyclic compounds like pyridine and pyrimidine derivatives are associated with antibacterial [3,4], antioxidant [5], antitumor [6,7], anticancer, fungicidal [8] and anti-inflammatory agents [9]. Some of the halogenated pyridines are used as anti-colorectal cancer compounds [10], anti-hypoglycemic and antihypertensive agents [11]. Due to the wide application of fluorinated pyridines and potency of fluorine atom, it was thought worthwhile to prepare a new fluorinated pyridine.
Experimental
All reagents were purchased from commercial sources and used without further purification. Melting point was determined in one end open capillary tube on a liquid paraffin bath and was not corrected. Reaction was carried out under an inert nitrogen atmosphere. Mass spectra, 1H-NMR and 13C-NMR spectra were recorded for the compound on Agilent Mass spectrometer, Bruker Avance II (399.65 MHz, 1H-NMR; 100.50 MHz, 13C-NMR) instruments, respectively. Chemical shifts were reported in parts per million (ppm) using tetramethylsilane (TMS) as an internal standard and Elemental (C, H and N) analysis was performed on an Elementar vario MICRO cube.
Synthesis of 6-[5-Chloro-2-(trifluoromethyl)phenyl]-3-fluoro-2-methylpyridine (3)
The title compound (3) was synthesized according to a known procedure reported for the synthesis of isomeric compound 6-[4-chloro-2-(trifluoromethyl)phenyl]-3-fluoro-2-methylpyridine [12] (Scheme 1.). A mixture of 5-fluoro-6-methylpyridin-2-ylboronic acid (1) (1.0 g, 6.49 mmol), 1,4-dioxane (16 mL) and water (8 mL) was prepared in a round bottom flask at room temperature under nitrogen atmosphere. The reaction mixture was degassed with argon for 15 min, K2CO3 (1.34 g, 9.74 mmol) and Bis(triphenylphosphine)palladium(II) dichloride (dikis) (0.22 g, 0.32 mmol) were added and degassed for 25 min. To this solution, 4-chloro-2-iodo-1-(trifluoromethyl)benzene (2) (1.95 g, 6.49 mmol) was added and the reaction mixture was heated for 6 h at 100 °C. It was allowed to cool to room temperature and diluted with ethyl acetate, filtered over celite, washed with ethyl acetate. The filtrate was washed with water and brine solution. Organic layer was dried over anhydrous MgSO4 and concentrated to give the crude product which was further purified by column chromatography using petroleum ether and ethyl acetate (7:3) as eluent to get the title compound (3) as a white solid with Rf = 0.64.
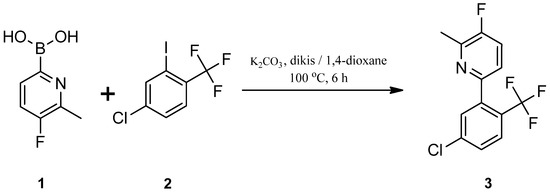
Scheme 1.
Synthetic route for the title compound, 6-[5-chloro-2-(trifluoromethyl)phenyl]-3-fluoro-2-methylpyridine (3).
Yield: 1.6 g (86%).
Melting point: 141–143 °C.
MS: m/z: 290.2 (M+ + 1).
IR: νmax/cm−1: 3091–2957 (aromatic C-H stretching), 1314–1221 (CF3 stretching), 1618 and 1431 (C=C stretching).
1H-NMR (CDCl3) δ ppm: 7.74 (d, J = 1.9 Hz 1H, Ar-H), 7.58 (dd, J = 8.3 Hz and J = 1.9 Hz 1H, Ar-H), 7.44–7.34 (m, 2H, Ar-H), 7.24–7.21 (m, 1H, Ar-H), 2.57 (d, J = 2.7, 3H, Ar-CH3).
13C-NMR (DMSO-d6): δ ppm: 158.6, 156.1, 151.5, 146.5, 137.8, 134.5, 133.0, 131.6, 126.6 CF3, 124.5, 122.6, 121.8, 17.9.
Elemental analysis: Calculated for: C13H8ClF4N: C, 53.91%; H, 2.78%; N, 4.84%. Found: C, 53.89%; H, 2.76%; N, 4.83%.
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3Acknowledgments
We gratefully acknowledge the guidance by S.C. Sharma, Former Vice Chancellor, Tumkur University, Tumkur.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Filler, R.; Saha, R. Flurine in medicinal chemistry: A century of progress and a 60-year retrospective of selected highlights. Future Med. Chem. 2009, 1, 777–791. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Ni, C.; Hu, J. Selective fluoroalkylation of organic compounds by tackling the “Negative Fluorine Effect”. Top Curr. Chem. 2012, 308, 25–44. [Google Scholar] [PubMed]
- Jian, W.; Shenghong, K.; Baoan, S.; Deyu, H.; Ming, H.; Linhong, J.; Song, Y. Synthesis and antibacterial activity against ralstonia solanacearum for novel hydrazone derivatives containing a pyridine moiety. Chem. Cent. J. 2012, 6, 1–5. [Google Scholar]
- Chavhan, N.M.; Badadhe, P.V.; Joshi, R.S.; Mandhane, P.G.; Gill, C.H. Synthesis and Biological Evaluation of Some Heterocycles from 1-Phenyl-3-(pyridine-3-yl)-1H-pyrazole-4-carbaldehyde. Asian J. Chem. 2010, 22, 4255–4260. [Google Scholar]
- Prachayasittikul, S.; Suksrichavalit, T.; Isarankura-Na-Ayudhya, C.; Ruchirawat, S.; Prachayasittikul, V. Antimicrobial and antioxidative activities of 1-adamantylthio derivatives of 3-substituted pyridines. EXCLI 2008, 7, 63–70. [Google Scholar]
- Ghorab, M.M.; Ragab, F.A.; Heiba, H.I.; Youssef, H.A.; El-Gazzar, M.G. Synthesis of novel pyrazole and pyrimidine derivatives bearing sulfonamide moiety as antitumor and radiosensitizing agents. Med. Chem. Res. 2012, 21, 1376–1386. [Google Scholar] [CrossRef]
- Zheng, Q.Z.; Zhang, X.M.; Xu, Y.; Cheng, K.; Jiao, Q.C.; Zhu, H.L. Synthesis, biological evaluation, and molecular docking studies of 2-chloropyridine derivatives possessing 1,3,4-oxadiazole moiety as potential antitumor agents. Bioorg. Med. Chem. 2010, 18, 7836–7841. [Google Scholar] [CrossRef] [PubMed]
- Tumkevicius, S.; Urbonas, A.; Vainilavicius, P. Synthesis of methyl esters of 5-amino-4-(substituted amino)-2-methylthio-7H-pyrrolol[2,3-d]-pyrimidine-6-carboxylic acids. Chem. Heterocycl. Compd. 2000, 36, 841–846. [Google Scholar] [CrossRef]
- Prasad, Y.R.; Rajasekhar, K.K.; Shankarananth, V.; Reddy, K.N.; Pradeepkumar, G.S.S.; Nagendrababu, R. Synthesis, antiinflammatory and antimicrobial evaluation of 2-phenyl-4,6-diaryl substituted pyrimidine derivatives. J. Pharm. Res. 2010, 3, 2480–2482. [Google Scholar]
- Gopalakrishnan, S.; Vadivel, E.; Pon Sathieshkumar, P.; Praba, M.S. Synthesis, molecular docking and ADME prediction of some pyridine and pyrimidine derivatives as anti-colorectal cancer drugs. J. Chem. Pharm. Res. 2010, 2, 60–66. [Google Scholar]
- Dolzhenko, A.V.; Kolotova, N.V.; Koz’minykh, V.O.; Syropyatov, B.Y.; Kotegov, V.P.; Godina, T. Substituted amides and hydrazides of dicraboxylic acids. Part 12.1 Synthesis and hypoglycemic and hypertensive activity of some pyridylamides and acylhydrazides of malic and citraconic acid. Pharm. Chem. J. 2002, 36, 174–176. [Google Scholar] [CrossRef]
- Sreenivasa, S.; Manojkumar, K.E.; Suchetan, P.A.; Mohan, N.R.; Palakshamurthy, B.S.; Srinivas, T.; Velmurgand, D. 6-[4-Chloro-2-(trifluoromethyl)phenyl]-3-fluoro-2-methylpyridine. Acta Cryst. 2012. [Google Scholar] [CrossRef] [PubMed]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).