Abstract
The title compound, 2-chloro-7-methyl-3-({4-[(4-nitrophenoxy)methyl]-1H-1,2,3-triazol-1-yl}methyl)quinoline was synthesized in one pot. The structure of the compound was fully characterized by IR, 1H and 13C-NMR, mass spectral analysis and elemental analysis.
Since the discovery of penicillin by Alexander Fleming and Prontosil by Gerald Domagk the arsenal of antibacterials available for the treatment of infectious disease has expanded exponentially [1]. 2-Chloroquinolines A (Scheme 1) represents an important class of bio active molecules that shows a wide range of pharmacological activities [2,3,4,5,6,7] including anti bacterial activity. On the other hand triazole core B (Scheme 1) is a structural motif of particular interest in the field of medicinal chemistry [8,9,10].
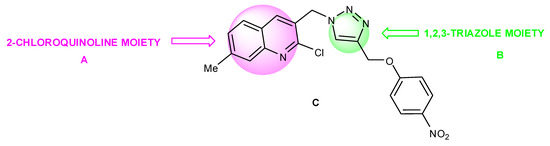
Scheme 1.
Design of hybrid molecule.
Because of their common vast range of biological activity and our interest in quinoline derivatives [11,12] novel 1,4 disubstituted 1,2,3 triazolyl-2-chloroquinoline C was designed having structural features of both A and B (Scheme 1) and an efficient synthesis was achieved in aqueous medium using copper catalyzed 1,3-dipolar [2+3] cycloaddition between azide and a terminal alkyne (CuAAC) [13]. We estimated that combination of structural features of triazole and 2-chloroquinoline would provide new promising pharmacological agent.
The starting compound 1 required for our study was prepared in 85% yield from 2-chloro-7-methylquinoline-3-carbaldehyde [2] via reduction of its aldehyde group to primary alcohol, using sodium borohydride in ethanol at room temperature followed by conversion of the hydroxyl group to chloro group by refluxing the solution in the presence of thionyl chloride and catalytic amount of pyridine in benzene (Scheme 2).
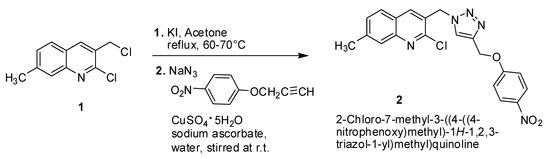
Scheme 2.
Synthesis of the title compound.
After synthesizing 2-chloro-3-(chloromethyl)-7-methylquinoline (1), we performed a one pot sequential reaction to get the desired target molecule. A mixture of compound 1 (0.904 g, 4 mmol), and potassium iodide (0.766 g, 4.6 mmol), in acetone (15 mL) was refluxed for 1.5 h and then cooled to room temperature. To this was added sodium azide (0.28 g, 4.4 mmol), 1-nitro-4-(prop-2-ynyloxy)benzene (0.779 g, 4.4 mmol), copper sulphate (0.996 g, 4 mmol), sodium ascorbate (0.396 g, 2 mmol), and water (10 mL). The mixture was stirred for 0.5 h at room temperature and the progress of the reaction was monitored by checking TLC using 3:1 chloroform: ethyl acetate as eluent in regular intervals. After completion of the reaction, the reaction mixture was filtered. The filtered solid was purified by column chromatography using 3:1 chloroform-ethyl acetate.
Description of the compound 1: White solid.
Yield: 95%.
M.p.: 120–122 °C.
Rf: 0.94 (Chloroform:Ethyl acetate = 1:1).
IR υmax (KBr cm−1): 3043, 3017, 2969, 2919, 1620, 1602, 1564, 1042, 699.
Mass (ES): m/z 226 (M+1, 100%).
1H-NMR (CDCl3, 400 MHz): δ 8.23 (s, 1H), 7.81 (s, 1H), 7.73 (d, J = 8.3 Hz, 1H), 7.43 (d, J = 8.3 Hz, 1H), 4.83 (s, 2H), 2.27 (s, 3H).
13C-NMR (CDCl3, 100 MHz): δ 149.6, 147.5, 141.8, 138.6, 129.8, 128.1, 127.2, 126.9, 125.1, 40.4, 20.2.
Elemental analysis found C, 58.55; H, 4.18; N, 6.29. Calc. for C11H9Cl2N; C, 58.43; H, 4.01; Cl, 31.36; N 6.19.
Description of the compound 2: Off white solid.
Yield: 1.54 g, 94%.
M.p.: 175–177 °C.
Rf: 0.39 (Chloroform:Ethyl acetate = 3:1).
IR υmax (KBr, cm−1): 3151, 3118, 2924, 2852, 1594, 1496.0, 1340, 1264, 1230, 1111.
Mass (ES): m/z 410 (M+1, 100%).
1H-NMR (CDCl3, 400 MHz): δ 8.20 (d, J = 9.3 Hz, 2H), 7.94 (s, 1H), 7.80 (s, 2H), 7.66 (d, 1H, J = 8.3 Hz), 7.42 (d, 1H, J = 8.3 Hz), 7.07 (d, 2H, J = 9.3 Hz), 5.82 (s, 2H), 5.32 (s, 2H), 2.57 (s, 3H).
13C-NMR (CDCl3, 100 MHz): δ 163.0, 148.9, 147.9, 142.7, 142.4, 141.9, 140.8, 138.8, 137.0, 130.2, 127.4, 125.9, 125.3, 123.5, 114.9, 62.4, 51.5, 27.5.
Elemental analysis found C, 58.46; H, 3.79; N, 17.38. Calc. for C20H16ClN5O3; C, 58.61; H, 3.94; Cl, 8.65; N, 17.09; O, 11.71.
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3Supplementary File 4Acknowledgements
The authors (S. Pal and K. S. S. Praveena) thank M. N. Raju for his constant encouragement.
References
- O’Meara, S.M.; Cullum, N.A.; Majid, M.; Sheldon, T.A. Systematic review of antimicrobial agents used for chronic wounds. Br. J. Surg. 2001, 88, 4–21. [Google Scholar] [CrossRef] [PubMed]
- Abdulqader, A.A.; Abdelkader, M.A.; Lien, E.J. Design, Synthesis, and pharmacological activities of 2-substituted 4-phenylquinolines as potential antidepressant drugs. J. Med. Chem. 1985, 28, 1394–1398. [Google Scholar]
- Kumar, S.; Bawa, S.; Drabu, S.; Kumar, R.; Panda, B.P. New 2-chloro-7-methylquinoline amine analogues as possible antimycotic agents. Lat. Am. J. Pharm. 2010, 29, 968–975. [Google Scholar]
- Kumar, S.; Bawa, S.; Gupta, H. Biological activities of quinoline derivatives. Mini Rev. Med. Chem. 2009, 14, 1648–1654. [Google Scholar] [CrossRef]
- Kumar, S.; Bawa, S.; Drabu, S.; Gupta, H.; Machwal, L.; Kumar, R. Synthesis, antidepressant and antifungal evaluation of novel 2-chloro-8-methylquinoline amine derivatives. Eur. J. Med. Chem. 2011, 46, 670–675. [Google Scholar] [CrossRef] [PubMed]
- Bindu, P.J.; Mahadevan, K.M.; Satyanarayan, N.D.; Ravikumar Naik, T.R. Synthesis and DNA cleavage studies of novel quinoline oxime esters. Bioorg. Med. Chem. Lett. 2012, 22, 898–900. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.; Rizvi, S.U.F.; Siddiqui, H.L.; Ahmad, S.; Parvez, M.; Suliman, R. Antioxidant and antimicrobial studies of novel N′-(substituted-2-chloroquinolin-3-yl)methylidene-4-hydroxy-2H-1,2-benzothiazine-3-carbohydrazides-1,1-dioxides. Med. Chem. Res. 2012, 21, 2340–2348. [Google Scholar] [CrossRef]
- Shafran, E.A.; Bakulev, V.A.; Rozin, Y.A.; Shafran, Y.M. Condensed 1,2,3-triazoles (review). Chem. Heterocycl. Comp. 2008, 44, 1040–1069. [Google Scholar] [CrossRef]
- Agalave, S.G.; Maujan, S.R.; Pore, V.S. 1,2,3-Triazole tethered β-lactam-chalcone bifunctional hybrids: Synthesis and anticancer evaluation. Eur. J. Med. Chem. 2012, 47, 594–600. [Google Scholar]
- Singh, P.; Raj, R.; Kumar, V.; Mahajan, M.P.; Bedi, P.M.S.; Kaur, T.; Saxena, A.K. Click chemistry: 1,2,3-Triazoles as pharmacophores. Chem. Asian J. 2011, 6, 2696–2718. [Google Scholar]
- Reddy, L.V.; Nakka, M.; Alishetty, S.; Ghosh, S.; Helliwell, M.; Khagga, M.; Mukherjee, A.K.; Pal, S. Synthesis of novel quinoline analogues of nimesulide: An unusual observation. J. Heterocycl. Chem. 2011, 48, 555–562. [Google Scholar] [CrossRef]
- Reddy, L.V.; Nallapati, S.B.; Beevi, S.S.; Mangamoori, L.S.; Khagga, M.; Pal, S. A "green" synthesis of N-(quinoline-3-ylmethylene)benzohydrazide derivatives and their cytotoxicity activities. J. Braz. Chem. Soc. 2011, 22, 1742–1749. [Google Scholar] [CrossRef]
- Himo, F.; Lovell, T.; Hilgraf, R.; Rostovtsev, V.V.; Noodleman, L.; Sharpless, K.B.; Fokin, V.V. Copper(I)-catalyzed synthesis of azoles. DFT study predicts unprecedented reactivity and intermediates. J. Am. Chem. Soc. 2005, 127, 210–216. [Google Scholar] [CrossRef] [PubMed]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).