Abstract
Diels Alder reaction of 4-bromophenylquinone (Br-PQ) with anthracene, followed by reduction affords the desired 4'-bromophenyl triptycene-2,5-diol (T-Br-PH). The described synthesis represents a simple and efficient method for the construction of a triptycene ring with a bromophenyl pendant. The intermediate and the final compound (T-Br-PH) have been characterized by elemental analysis, NMR, and LCMS techniques.
4-Bromophenylquinone (Br-PQ) was synthesized by the reaction among 4-bromo aniline and benzoquinone [1]. The triptycene ring creation was achieved by the Diels Alder reaction [2,3,4]. In the course of our work on the synthesis, first we planned and synthesized Br-PQ in the typical method already reported; and finally the target molecule (Scheme 1).
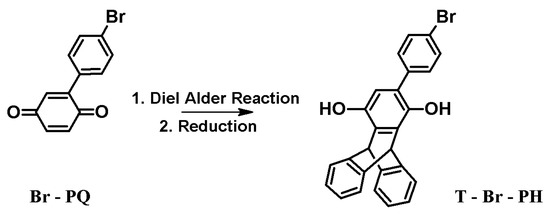
Scheme 1.
Synthetic pathway to afford T-Br-PH.
In recent years, due to the number of practical applications in the field of liquid crystalline materials and liquid crystalline polymers [5,6], a great deal of interest has been shown in the study of the rigid “paddlewheel” structured triptycene [7], incorporation of these rigid pendant groups will substantially improve the mechanical properties of the polymers [8,9]. Polymers having bromine functional group can be ardently convert in to various functionalities in addition to hydroxyl moiety [10,11], there are adequately innumerable derivatives can be constructed. In order to fulfill the requirements of the polymer as well as material science field, the reported target molecule as monomer can constrain their application in the large scale.
Experimental
Compound Br-PQ is not commercially available, and procedure is adopted to synthesize as previously described [1].
Step 1: Diel’s Alder Reaction
The mixture of Br-PQ (0.25g, 0.95mmol) and anthracene (0.17 g, 0.95 mmol) was refluxed in xylene for 10 h, the progress of the reaction was monitored by TLC (15% ethyl acetate in petroleum ether), and the solvent was evaporated. The crude compound (0.38 g) was repeatedly recrystallized with toluene and recrystallized compound (0.31 g) taken for next step.
Step 2: Reduction
The step 1 compound (0.31 g, 0.7 mmol) was suspended in diethylether solvent and sodium di thionate (0.3 g, 1.8 mmol) was added in portion wise, the reaction mixture was stirred at room temperature for 1 h. The reduced crude compound was obtained after the evaporation of solvent under reduced pressure, the crude product was purified using column chromatography by petrolium ether and ethyl acetate as eluent, and product was eluted at 32% of ethyl acetate. Orange crystals were afforded after the removal of solvent in rotary evaporator (0.18 g, 60%); the separated solids were vacuum dried to remove residual solvent impurities.
Br-PQ
1H-NMR (400 MHz, DMSO-d6): δ = 7.63 (d, J = 8 Hz, 2H), 7.46 (d, J = 8 Hz, 2H), 6.95 (d, J = 7.2 Hz, 1H), 6.90 (d, J = 10 Hz, 1H), 6.68 (s, 1H).
13C-NMR (100 MHz, DMSO-d6): 150.65, 147.15, 138.44, 131.53, 131.26, 127.21, 120.15, 117.45, 116.61, 115.97.
LCMS: m/z (ES), 262.1 [(M−1)−].
Elemental analysis: Calculated for C12H7BrO2 (263.08): C, 54.78%; H, 2.68%; O, 12.16%. Found: C, 54.52%; H, 2.48%; O, 12.02%.
T-Br-PH
M.p. 118.0–119.6 °C
1H-NMR (400 MHz, DMSO-d6): δ = 9.09 (s, 1H), 8.40 (s, 1H), 8.41 (s, 1H), 7.52 (dd, J = 8.4 Hz and 2 Hz, 2H), 7.40 (dd, J = 8.4 Hz and 2 Hz, 4H), 7.32 (dd, J = 8.4 Hz and 2.8 Hz, 2H), 6.97 (dd, J = 8.4 Hz and 2 Hz, 4H). 6.36 (s, 1H), 5.99 (s, 1H), 5.82 (s, 1H).
13C-NMR (100 MHz, DMSO-d6): 146.11, 145.96, 141.72, 138.67, 135.08, 132.29, 131.72, 127.03, 125.28, 125.19, 124.21, 124.03, 120.18, 114.41, 60.20, 47.40, 47.09, 21.21 and 14.55.
MS: m/z (ES), 440.2 [(M−1)−].
Elemental analysis: Calculated for C26H17BrO2 (441.31): C, 70.76%; H, 3.88%; O, 7.25%. Found: C, 70.62%; H, 3.76%; O, 7.17%.
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3Supplementary File 4Acknowledgements
One of the authors (Shimoga D. Ganesh) wishes to acknowledge the UGC, New Delhi, for awarding the Research Fellowship under meritorious category.
References
- Liu, B.; Robertson, G.P.; Guiver, M.D.; Shi, Z.; Navessin, T.; Holdcroft, S. Fluorinated poly(aryl ether) containing a 4-bromophenyl pendant group and its phosphonated derivative. Macromol. Rapid Commun. 2006, 27, 1411–1417. [Google Scholar] [CrossRef]
- Alonso, M.Á.; Alvarado, P.L.; Avendaño, C.; Menéndez, C.J. Regioselective Diels-Alder reactions of 3-vinylindoles with quinones. Lett. Org. Chem. 2004, 1, 20–22. [Google Scholar] [CrossRef]
- Taylor, M.S.; Swager, T.M. Triptycenediols by rhodium-catalyzed [2+2+2] cycloaddition. Org. Lett. 2007, 9, 3695–3697. [Google Scholar] [CrossRef] [PubMed]
- Thomas, S.W., III; Long, T.M.; Pate, B.D.; Kline, S.R.; Thomas, E.L.; Swager, T.M. Perpendicular organization of macromolecules: Synthesis and alignment studies of a soluble poly(iptycene). J. Am. Chem. Soc. 2005, 127, 17976–17977. [Google Scholar] [CrossRef] [PubMed]
- Long, T.M.; Swager, T.M. Triptycene-containing bis(phenylethynyl)benzene nematic liquid crystals. J. Mater. Chem. 2002, 12, 3407–3412. [Google Scholar] [CrossRef]
- Song, M.H.; Park, B.; Nishimura, S.; Toyooka, T.; Chung, I.J.; Takanishi, Y.; Ishikawa, K.; Takezoe, H. Electrotunable non-reciprocal laser emission from a liquid-crystal photonic device. Adv. Funct. Mater. 2006, 16, 1793–1798. [Google Scholar] [CrossRef]
- Chmiel, J.; Heesemann, I.; Mix, A.; Neumann, B.; Stammler, H.G.; Mitzel, N.W. The effect of bulky substituents on the formation of symmetrically trisubstituted triptycenes. Eur. J. Org. Chem. 2010, 20, 3897–3907. [Google Scholar] [CrossRef]
- Swager, T.M. Iptycenes in the design of high performance polymers. Acc. Chem. Res. 2008, 41, 1181–1189. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Li, Z.; Wirth, T. Triptycene derivatives: Synthesis and applications. Chem. Lett. 2010, 39, 658–661. [Google Scholar] [CrossRef]
- Vacek, J.; Michl, J. Artificial surface-mounted molecular rotors: Molecular dynamics simulations. Adv. Funct. Mater. 2007, 17, 730–739. [Google Scholar] [CrossRef]
- Ghanem, B.S.; Msayib, K.J.; McKeown, N.B.; Harris, K.D.M.; Pan, Z.; Budd, P.M.; Butler, A.; Selbie, J.; Book, D.; Walton, A. A triptycene-based polymer of intrinsic microposity that displays enhanced surface area and hydrogen adsorption. Chem. Commun. 2007, 67–69. [Google Scholar] [CrossRef] [PubMed]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).