Abstract
S-Alkylation of 5-(diphenylmethyl)-1,3,4-oxadiazole-2(3H)-thione (3) by 2-chloro-N-(pyrazin-2-yl)acetamide (2) affords the title compound, 2-{[5-(diphenylmethyl)-1,3,4-oxadiazol-2-yl]sulfanyl}-N-(pyrazin-2-yl)acetamide (4). The intermediate (2), in turn, was prepared by the acetylation of 2-aminopyrazine (1) with chloroacetyl chloride. The structure of the newly synthesized compound is characterized by IR, NMR and mass spectral data.
Introduction
Thioethers have emerged as preeminent classes of organic compounds, which hold useful applications as key reagents in organic synthesis, bioorganic, medicinal, and heterocyclic chemistry [1,2,3,4]. Thioether derivatives have been the subject of investigation due to their different properties such as antibacterial [5] and antifungal [6] activities. Literature survey reveals that pyrazine derivatives showed pronounced antiauxin [7], antimycobacterial [8], antimicrobial, and anticancer [9] activities. On the other hand, a wide range of biological activities like, antiinflammatory [10], analgesic [11], antimicrobial [12,13] etc., have been attributed to the compounds containing 1,3,4-oxadiazole ring system [14,15,16,17]. In view of the importance of thioethers, pyrazines and 1,3,4-oxadiazoles, it was aimed to synthesize the title compound, with the combination of three pharmacophore groups.
Results and Discussion
The title compound, 2-{[5-(diphenylmethyl)-1,3,4-oxadiazol-2-yl]sulfanyl}-N-(pyrazin-2-yl)acetamide (4), was prepared by the regioselective S-alkylation of 5-(diphenylmethyl)-1,3,4-oxadiazole-2(3H)-thione (3) with 2-chloro-N-(pyrazin-2-yl)acetamide (2) (Scheme 1). The intermediate 2, in turn, was prepared by the reaction of 2-aminopyrazine (1) with 2-chloroacetylchloride in the presence potassium carbonate [16]. The other intermediate, 5-(diphenylmethyl)-1,3,4-oxadiazole-2(3H)-thione (3) was synthesized from diphenyl acetic acid according to the method described in our earlier work [17]. The title compound (4) was characterized by NMR, IR and mass spectral data.
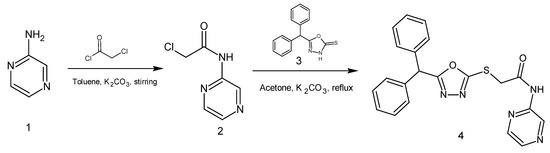
Scheme 1.
Synthesis of 2-{[5-(diphenylmethyl)-1,3,4-oxadiazol-2-yl]sulfanyl}-N-(pyrazin-2-yl) acetamide, 4.
The formation of 2-{[5-(diphenylmethyl)-1,3,4-oxadiazol-2-yl]sulfanyl}-N-(pyrazin-2-yl)acetamide (4) was confirmed by its NMR and mass spectral data. A singlet integrating for two protons at δ 4.53 in the 1H-NMR spectrum of compound (4) was due to -S-CH2 linkage. Another singlet observed at δ 5.93 ppm was due to the proton attached the carbon for which two phenyl groups were substituted. The signals of aromatic protons merged in the regions δ 7.22–7.36 ppm and 8.38–8.41 ppm as multiplets. NH proton appeared as a singlet at δ 11.15 ppm as a singlet. Mass spectrum showed a molecular ion peak at m/z 404.5 (M++1) corresponding to the molecular formula of C21H17N5O2S. Elemental analysis and 13C-NMR spectrum also gave satisfactory results for the title compound.
Experimental
The melting point was taken in an open capillary tube and was uncorrected. The purity of the compound was confirmed by thin layer chromatography using Merck silica gel 60 F254 coated aluminium plates. IR spectrum was recorded on Shimadzu-FTIR Infrared spectrometer in KBr (νmax in cm−1). 1H-NMR (400 MHz) spectrum was recorded on a Varian 400 spectrometer, with 5 mm PABBO BB-1H TUBES and 13C-NMR (100 MHz) spectrum was recorded for approximately 0.03 M solutions in DMSO at 100 MHz with TMS as internal standard. LCMS was obtained using an Agilent 1200 series LC and Micromass zQ spectrometer. Elemental analysis was carried out by using VARIO EL-III (Elementar Analysensysteme GmBH).
The synthesis of 2-chloro-N-(pyrazin-2-yl)acetamide (2) was carried out by the reaction of 2-aminopyrazine (1) with 2-chloroacetyl chloride in the presence of K2CO3 [18]. The other intermediate, 5-(diphenylmethyl)-1,3,4-oxadiazole-2(3H)-thione 3 was synthesized by adopting the method described in our earlier work [19].
To a solution of 5-(diphenylmethyl)-1,3,4-oxadiazole-2(3H)-thione 3 (0.44 g, 1.65 mmol) in dry acetone (25 mL), anhydrous potassium carbonate (0.27 g, 1.98 mmol) and 2-chloro-N-(pyrazin-2-yl)acetamide 2 (0.34 g, 1.98 mmol) were added and the resulting solution was heated to reflux for 4 hours. After the completion of reaction as indicated by TLC, the reaction mixture was cooled to room temperature and quenched into ice cold water. The resulting precipitate was filtered and recrystallized from ethanol. The yield was 0.73 g, 71%.
Melting point: 210–212 °C.
LCMS: m/z = 404.5 (M++1).
IR (KBr): νmax (cm−1), 3201 (N-H), 3041 (Ar-H), 1699 (alkyl-CO-NH) , 1555 (C=N, Ar C=C).
1H-NMR (400 MHz, DMSO-d6): δ ppm, 4.53 (s, 2H, S-CH2), 5.93 (s, 1H, Ph2-CH), 7.22–7.36 (m, 11H, Ar-H), 8.38–8.41 (m, 2H, Ar-H), 11.15 (s, 1H, NH).
13C-NMR (100 MHz, DMSO-d6,): δ ppm, 168.52 (C=O), 166.41, 163.89 (oxadiazole C’s), 148.75, 143.11, 140.54, 139.07, 136.55, 129.14, 128.75, 127.82 (aromatic C’s), 47.61(S-CH2), 36.78 (Ph2-CH).
Elemental analysis: Calculated for C21H17N5O2S, C, 62.52%; H, 4.25%; N, 17.36%; Found: C, 62.49%; H, 4.28%; N, 17.32%.
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3Supplementary File 4Acknowledgments
The authors are thankful to IISc, Bangalore for NMR data. BN thanks the UGC for financial assistance through BSR one time grant for the purchase of chemicals. PSN thanks Mangalore University for research facilities and DST-PURSE for financial assistance.
References
- Cremlyn, R.J.W. An Introduction to Organosulfur Chemistry; Wiley: New York, NY, USA, 1996. [Google Scholar]
- Oae, S.; Okuyama, T. (Eds.) Organic Sulfur Chemistry: Biochemical Aspects; CRC: Boca Raton, FL, USA, 1992.
- Weinshilboum, R. Thiol S-methyltransferases, II: Pharmacogenetics. In Sulfur-Containing Drugs and Related Compounds Chemistry, Biochemistry and Toxicology; Damani, L.A., Ed.; Ellis Horwood: Chichester, UK, 1989; Volume 1, Part A, Chapter 1. [Google Scholar]
- McReynolds, M.D.; Doughtery, J.M.; Hanson, P.R. Synthesis of phosphorous and sulfur heterocycles via ring-closing metathesis. Chem. Rev. 2004, 104, 2239–2258. [Google Scholar] [CrossRef] [PubMed]
- Macaev, F.; Rusu, G.; Pogrebnoi, S.; Gudima, A.; Stingaci, E.; Vlad, L.; Shvets, N.; Kandemirli, F.; Dimoglo, A.; Reynolds, R. Synthesis of novel 5-aryl-2-thio-1,3,4-oxadiazoles and the study of their structure–anti-mycobacterial activities. Bioorg. Med. Chem. 2005, 13, 4842–4850. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Li, Z.; Han, Y. Synthesis and fungicidal activity against rhizoctonia solani of 2-alkyl (alkylthio)-5-pyrazolyl-1,3,4-oxadiazoles (thiadiazoles). J. Agric. Food Chem. 2000, 48, 5312–5315. [Google Scholar] [CrossRef] [PubMed]
- Camper, N.D.; McDonald, S.K. Tissue and cell cultures as model system in herbicide research. Rev. Weed Sci. 1989, 4, 169–190. [Google Scholar]
- Dolezal, M.; Cmedlova, P.; Palek, L.; Vinsova, J.; Kunes, J.; Buchta, V.; Jampilek, J.; Kralova, K. Synthesis and antimycobacterial evaluation of substituted pyrazinecarboxamides. Eur. J. Med. Chem. 2008, 43, 1105–1113. [Google Scholar] [CrossRef] [PubMed]
- Carta, A.; Sanna, P.; Gherardini, L.; Usai, D.; Zanetti, S. Novel functionalized pyrido[2,3-g]quinoxalinones as antibacterial, antifungal and anticancer agents. II Farmaco 2001, 56, 933–938. [Google Scholar] [CrossRef]
- Narayana, B.; Ashalatha, B.V.; Vijaya Raj, K.K.; Fernandes, J.; Sarojini, B.K. Synthesis of some new biologically active 1,3,4-oxadiazolyl nitroindoles and a modified Fischer indole synthesis of ethyl nitro indole-2-carboxylates. Bioorg. Med. Chem. 2005, 13, 4638–4644. [Google Scholar] [CrossRef] [PubMed]
- Narayana, B.; Vijaya Raj, K.K.; Ashalatha, B.V.; Kumari, N.S. Synthesis of some new 2-(6 methoxy-2-naphthyl)- 5-aryl-1,3,4-oxadiazoles as possible non-steroidal anti-inflammatory and analgesic agents. Arch. Pharm. (Weinheim) 2005, 338, 373–377. [Google Scholar] [CrossRef] [PubMed]
- Mayekar, A.N.; Yathirajan, H.S.; Narayana, B.; Sarojini, B.K.; Kumari, N.S. Synthesis and antimicrobial studies on new substituted 1,3,4-oxadiazole derivatives bearing 6-bromo naphthalene moiety. Int. J. Chem. 2010, 2, 38–54. [Google Scholar] [CrossRef]
- Bharadwaj, N.; Sara, S.K.; Sharma, P.; Kumar, P. Synthesis, evaluation and characterization of some 1,3,4-oxadiazoles as antimicrobial agents. Eur. J. Chem. 2009, 6, 1133–1138. [Google Scholar]
- Hall, A.; Brown, S.H.; Chowdhury, A.; Giblin, G.M.P.; Gibson, M.; Healy, M.P.; Livermore, D.G.; Wilson, R.J.M.; Naylor, A.; Rawlings, D.A.; et al. Identification and optimization of novel 1,3,4-oxadiazole EP1 receptor antagonists. Bioorg. Med. Chem. Lett. 2007, 17, 4450–4455. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Hamid, M.K.; Abdel-Hafez, A.A.; El-Koussi, N.A.; Mahfouz, N.M.; Innocenti, A.; Supuran, C.T. Design, synthesis, and docking studies of new 1,3,4-thiadiazole-2-thione derivatives with carbonic anhydrase inhibitory activity. Bioorg. Med. Chem. 2007, 15, 6975–6984. [Google Scholar] [CrossRef] [PubMed]
- Amir, M.; Kumar, H.; Javed, S.A. Synthesis and pharmacological evaluation of condensed heterocyclic 6-substituted-1,2,4-triazolo[3,4-b]-1,3,4-thiadiazole derivatives of naproxen. Bioorg. Med. Chem. Lett. 2007, 17, 4504–4508. [Google Scholar] [CrossRef] [PubMed]
- Pastorin, G.; da Ros, T.; Bolcato, C.; Montopoli, C.; Moro, S.; Cacciari, B.; Baraldi, P.G.; Varani, K.; Borea, P.A.; Spalluto, G. Synthesis and biological studies of a new series of 5-heteroaryl carbamoylaminopyrazolo[4,3-e]1,2,4-triazolo[1,5-c]pyrimidines as human A3 adenosine receptor antagonists. Influence of the heteroaryl substituent on binding affinity and molecular modeling investigations. J. Med. Chem. 2006, 49, 1720–1729. [Google Scholar] [PubMed]
- Hassan, G.S.; El-Messery, S.M.; Al-Omary, F.A.M.; El-Subbagh, H.I. Substituted thiazoles VII. Synthesis and antitumor activity of certain 2-(substituted amino)-4-phenyl-1,3-thiazole analogs. Bioorg. Med. Chem. Lett. 2012, 22, 6318–6323. [Google Scholar] [CrossRef] [PubMed]
- Samshuddin, S.; Narayana, B.; Sarojini, B.K.; Shetty, D.N.; Kumari, N.S. Synthesis, characterization, and biological evaluation of some new functionalized terphenyl derivatives. Int. J. Med. Chem. 2012, 2012. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).