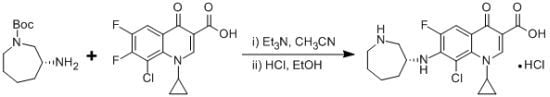
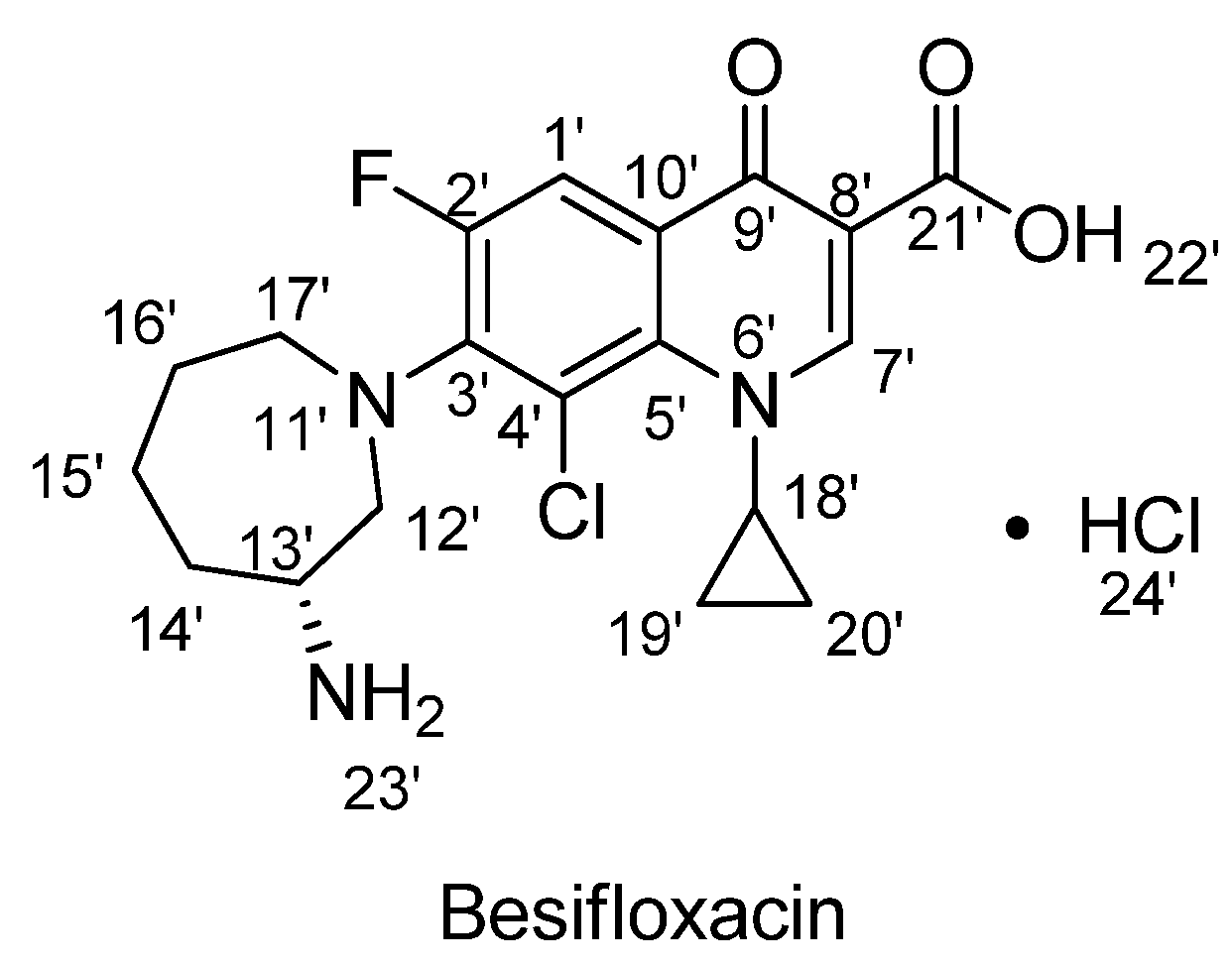
(R)-7-(Azepan-3-ylamino)-8-chloro-1-cyclopropyl-6-fluoro-4-oxo-1,4-dihydroquinoline-3-carboxylic Acid Hydrochloride
Abstract
:Experimental
Structural Characterization
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3Supplementary File 4Acknowledgements
References and Notes
- Haas, W.; Pillar, C.M.; Zurenko, G.E.; Lee, J.C.; Brunner, L.S.; Morris, T.W. Besifloxacin, a novel fluoroquinolone, has broad-spectrum in vitro activity against acrobic and anaerobic bacteria. Antimicrob Agents Chemother. 2009, 53, 3552–3560. [Google Scholar] [CrossRef] [PubMed]
- Tepedino, M.E.; Heller, W.H.; Usner, D.W.; Brunner, L.S.; Morris, T.W.; Haas, W.; Paterno, M.R.; Comstock, T.L. Phase III efficacy and safety study of besifloxacin ophthalmic suspension 0.6% in the treatment of bacterial conjunctivitis. Curr. Med. Res. Opin. 2009, 25, 1159–1169. [Google Scholar] [CrossRef] [PubMed]
- Karpecki, P.; DePaolis, M.; Hunter, J.A.; White, E.M.; Rigel, L.; Brunner, L.S.; Usner, D.W.; Paterno, M.R.; Comstock, T.L. Besifloxacin ophthalmic suspension 0.6% in patients with bacterial conjunctivitis: A multicenter, prospective, randomized, double-masked, vehicle-controlled, 5-day efficacy and safety study. Clin. Ther. 2009, 31, 514–526. [Google Scholar] [CrossRef] [PubMed]
- McDonald, M.B.; Protzko, E.E.; Brunner, L.S.; Morris, T.W.; Haas, W.; Paterno, M.R.; Comstock, T.L.; Usner, D.W. Efficacy and safety of besifloxacin ophthalmic suspension 0.6% compared with moxifloxacin ophthalmic solution 0.5% for treating bacterial conjunctivitis. Ophthalmology 2009, 116, 1615–1623. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, J.; Kim, A.; Pratzer, K.A.; Stark, W.J. Aqueous penetration of moxifloxacin 0.5% ophthalmic solution and besifloxacin 0.6% ophthalmic suspension in cataract surgery patients. J. Cataract Refract. Surg. 2010, 36, 1499–1502. [Google Scholar] [CrossRef] [PubMed]
- Harms, A.E.; Ramakrishnan, A.; Soni, A.K. Quinolone Carboxylic Acids, Derivatives Thereof, and Methods of Making and Using Same. U.S. Patent 8252783, 17 September 2009. [Google Scholar]
- Pellegata, R.; Pinza, M.; Pifferi, G. An improved synthesis of γ-, δ-, and ε-lactams. Synthesis 1978, 1978, 614–616. [Google Scholar] [CrossRef]
- Boyle, W.J.; Sifniades, S.; Peppen, J.F. Asymmetric transformation of α-amino-ε-caprolactam, a ysine precursor. J. Org. Chem. 1979, 44, 4841–4847. [Google Scholar] [CrossRef]
- Irakura, T.; Suzue, S.; Murayama, S.; Hirai, K.; Ishizaki, T. Quinolinecarboxylic Acid Derivatives and Process for Their Preparation. Eur. Pat. Appl. 0195841, 1 October 1986. [Google Scholar]
- Sanchez, J.P.; Domagala, J.M.; Hagen, S.E.; Heifetz, C.L.; Hutt, M.P.; Nichols, J.B.; Trehan, A.K. Quinolone antibacterial agents. Synthesis and structure-activity relationships of 8-substituted quinoline-3-carboxylic acids and 1,8-naphthyridine-3-carboxylic acids. J. Med. Chem. 1988, 31, 983–991. [Google Scholar] [CrossRef] [PubMed]

© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Xia, Z.; Chen, Z.; Yu, S. (R)-7-(Azepan-3-ylamino)-8-chloro-1-cyclopropyl-6-fluoro-4-oxo-1,4-dihydroquinoline-3-carboxylic Acid Hydrochloride. Molbank 2013, 2013, M801. https://doi.org/10.3390/M801
Xia Z, Chen Z, Yu S. (R)-7-(Azepan-3-ylamino)-8-chloro-1-cyclopropyl-6-fluoro-4-oxo-1,4-dihydroquinoline-3-carboxylic Acid Hydrochloride. Molbank. 2013; 2013(2):M801. https://doi.org/10.3390/M801
Chicago/Turabian StyleXia, Zhengjun, Zaixin Chen, and Shuitao Yu. 2013. "(R)-7-(Azepan-3-ylamino)-8-chloro-1-cyclopropyl-6-fluoro-4-oxo-1,4-dihydroquinoline-3-carboxylic Acid Hydrochloride" Molbank 2013, no. 2: M801. https://doi.org/10.3390/M801
APA StyleXia, Z., Chen, Z., & Yu, S. (2013). (R)-7-(Azepan-3-ylamino)-8-chloro-1-cyclopropyl-6-fluoro-4-oxo-1,4-dihydroquinoline-3-carboxylic Acid Hydrochloride. Molbank, 2013(2), M801. https://doi.org/10.3390/M801