Abstract
Novel 4-[(2-hydroxy-4-pentadecylbenzylidene)amino]-1,5-dimethyl-2-phenyl-1,2-dihydro-3H-pyrazol-3-one was prepared via condensation of 2-hydroxy-4-pentadecylbenzaldehyde (1) with 4-amino-1,2-dihydro-2,3-dimethyl-1-phenylpyrazol-5-one (2) in ethanol/acetic acid under reflux. The structure of the synthesized compound was assigned on the basis of elemental analysis and spectral data.
Introduction
Pyrazolone and its derivatives form an important class of organic compounds due to their structural chemistry and biological activities as HIV-1 integrase inhibitors, anticancer, anti-inflammatory, antipyretics and anti-oxidant [1,2,3,4,5]. Even the simplest pyrazolone derivatives like antipyrine and amidopyrine are widely used analgesic medicines [6]. In continuation of previous work on 2-hydroxy-4-pentadecylbenzaldehyde compound [7], we are reporting here in the synthesis of 4-[(2-hydroxy-4-pentadecylbenzylidene)amino]-1,5-dimethyl-2-phenyl-1,2-dihydro-3H-pyrazol-3-one.
Result and Discussion
Cardanol is a mixture of natural alkyl phenols obtained by vacuum distillation of cashew nut shell liquid (CNSL). The structure and composition of cardanol is given in Figure 1. It is a mixture of saturated and unsaturated (mono-, di-, and tri-) compounds.
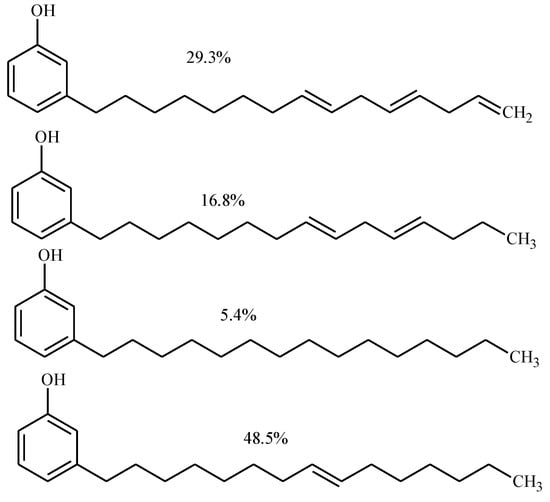
Figure 1.
Structure and composition of cardanol.
Cardanol (1) is a phenolic compound with a C15 aliphatic chain in the meta position, obtained from cashew nut shell liquid (CNSL) and then formylation of hydrogenated cardanol using Reimer-Tiemann reaction following a standard procedure [8] Figure 2.
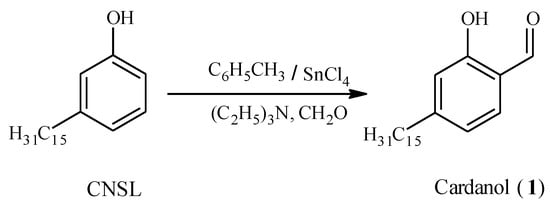
Figure 2.
Synthesis of cardanol.
Experimental
Melting point was determined in open capillary and is uncorrected. Absorption spectrum was recorded in CHCl3 by a Hewlett Packard-8453 spectrophotometer. FT-IR spectrum was recorded on a Nicolet Fourier Transform Infrared Spectrophotometer: Impact 410 (Nicolet Instrument Technologies, Inc. WI, USA). 1H-NMR and 13C-NMR were obtained in DMSO-d6 at 500 MHz for 1H nuclei and 125 MHz for 13C nuclei (Bruker Company, Germany). All chemical shifts were reported in parts per million (ppm) using residual proton or carbon signal in deuterated solvents as internal references. Mass spectrum was obtained using matrix-assisted laser desorption ionization mass spectrometry (MALDI-TOF) by using dithranol as a matrix. Elemental analysis (C, H and N) was performed on a Perkin Elmer 2400 analyzer. The purity of the compound was checked by TLC on silica gel and further purification was performed through column chromatography (silica gel, 60–120 mesh).
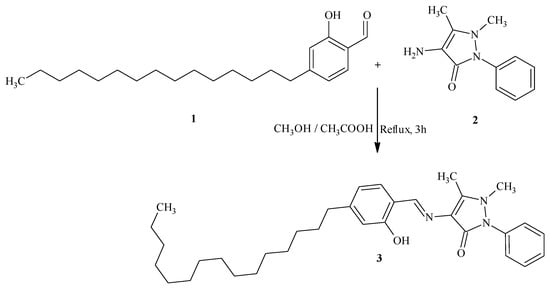
A mixture of 2-hydroxy-4-pentadecylbenzaldehyde 1 (1.10 g, 0.003 mol) and 4-amino-1,2-dihydro-2,3-dimethyl-1-phenylpyrazol-5-one 2 (0.60 g, 0.003 mol) in methanol (15 mL) in the presence of glacial acetic acid (1 mL) was refluxed for 3h. After the completion of the reaction (TLC-monitoring), the reaction mixture was cooled down to room temperature and then poured into crushed ice. The precipitate was filtered, dried and recrystallized from methanol. The solid was further purified through column chromatography [silica, petroleum ether/ethyl acetate (50:50)], leading to compound 3 as yellow solid.
Yield: 0.90 g (84%).
Melting point: 236–238 °C.
λabs = 350 nm.
LC-MS: m/z = 517.93 (M+).
IR (KBr): νmax (cm−1): 3435.69 (O-H str.), 1644.77 (C=O str.), 1595.15 (C=N str.).
1H-NMR (500 MHz, DMSO-d6) δ ppm: 12.93 (s, 1H, OH), 9.64 (s, 1H, CH=N), 7.54 (d, J = 7.8 Hz, 1H, Ar-H), 7.52 (d, J = 7.5 Hz, 1H, Ar-H), 7.37 (m, 3H, Ar-H), 7.33 (d, J = 7.7 Hz, 1H, Ar-H), 6.73 (d, J = 7.6 Hz, 1H, Ar-H), 6.71 (s, 1H, Ar-H), 3.18 (s, 3H, N-CH3), 2.53 (t, J = 7.6 Hz, 2H, Ar-CH2), 2.38 (s, 3H, C-CH3), 1.56–1.13 (m, 26H, (CH2)13), 0.83 (t, J = 6.8 Hz, 3H, CH3).
13C-NMR (125 MHz, DMSO-d6) δ ppm: 157.2, 143.6 (CH=N), 135.0, 132.5, 132.2, 131.8, 131.6, 129.2, 129.0, 125.9, 124.7, 118.8, 115.0, 112.5 (Aromatic carbons), 35.1, 31.2, 30.7, 30.5, 28.9, 28.8, 28.6, 28.6, 28.4, 22.0 (Methylene carbons), 13.8 (CH3).
Elemental analysis: Calculated for C33H47N3O2: C, 76.55%; H, 9.15%; N, 8.12%; found: C, 77.43%; H, 9.10%; N, 8.03%.
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3Acknowledgments
This work was supported by the Higher Education Research Promotion and National Research University Project of Thailand, Office of the Higher Education Commission (Project No. EN1250B). The post doctoral fellowship grant from the Ratchadapisakesompote Endownment Fund, Chulalongkorn University (G.N.) was gratefully acknowledged.
References
- Hadi, V.; Koh, Y.-H.; Sanchez, T.W.; Barrios, D.; Neamati, N.; Jung, K.W. Development of the next generation of HIV-1 integrase inhibitors: Pyrazolone as a novel inhibitor scaffold. Bioorg. Med. Chem. 2010, 20, 6854–6857. [Google Scholar] [CrossRef] [PubMed]
- Brana, M.F.; Gradillas, A.; Ovalles, A.G.; Lopez, B.; Acero, N.; Llinares, F.; Mingarro, D.M. Synthesis and biological activity of N,N-dialkylaminoalkyl substituted bisindolyl and diphenyl pyrazolone derivatives. Bioorg. Med. Chem. 2006, 14, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Ismail, M.M.F.; Ammar, Y.A.; El-Zahaby, H.S.A.; Eisa, S.I.; Barakat, S.E. Synthesis of novel 1-pyrazolylpyridin-2-ones as potential anti-inflammatory and analgesic agents. Arch. Pharm. Life Sci. 2007, 340, 476–482. [Google Scholar] [CrossRef] [PubMed]
- El-Hawash, A.M.; El-Sayed, A.M.B.; El-Ashmawey, I.M. Nonsteroidal anti-inflammatory agents-part-2 anti-inflammatory, analgesic and antipyretic activity of some substituted 3-pyrazolin- 5-ones and 1,2,4,5,6,7-3H-hexahydroindazol-3-ones. Eur. J. Med. Chem. 2006, 41, 155–165. [Google Scholar] [CrossRef] [PubMed]
- Manojkumar, P.; Ravi, T.K.; Gopalakrishnan, S. Antioxidant and antibacterial studies of arylazopyrazoles and arylhydrazonopyrazolones containing coumarin moiety. Eur. J. Med. Chem. 2009, 44, 4690–4694. [Google Scholar] [CrossRef] [PubMed]
- Filho, V.C.; Correa, R.; Vaz, Z.; Calixto, J.B.; Nunes, R.J.; Pinheiro, T.R.; Andricopulo, A.D.; Yunes, R.A. Further studies on analgesic activity of cyclic imides. Farmaco 1998, 53, 55–57. [Google Scholar] [CrossRef]
- Gadada, Naganagowda; Amorn, Petsom. 4-Amino-N-(2-hydroxy-4-pentadecylbenzylidene) benzenesulfonamide. Molbank 2011, 2011, M739. [Google Scholar]
- Payne, P.; Tyman, J.H.P.; Mehet, S.K.; Ninagawa, A. The synthesis of 2-hydroxymethyl derivatives of phenols. J. Chem. Res. 2006, 37, 402–405. [Google Scholar] [CrossRef]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).