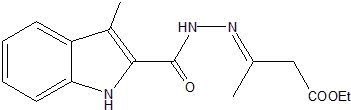
Ethyl 3-{2-[(3-Methyl-1H-indol-2-yl)carbonyl]hydrazinylidene}butanoate
Abstract
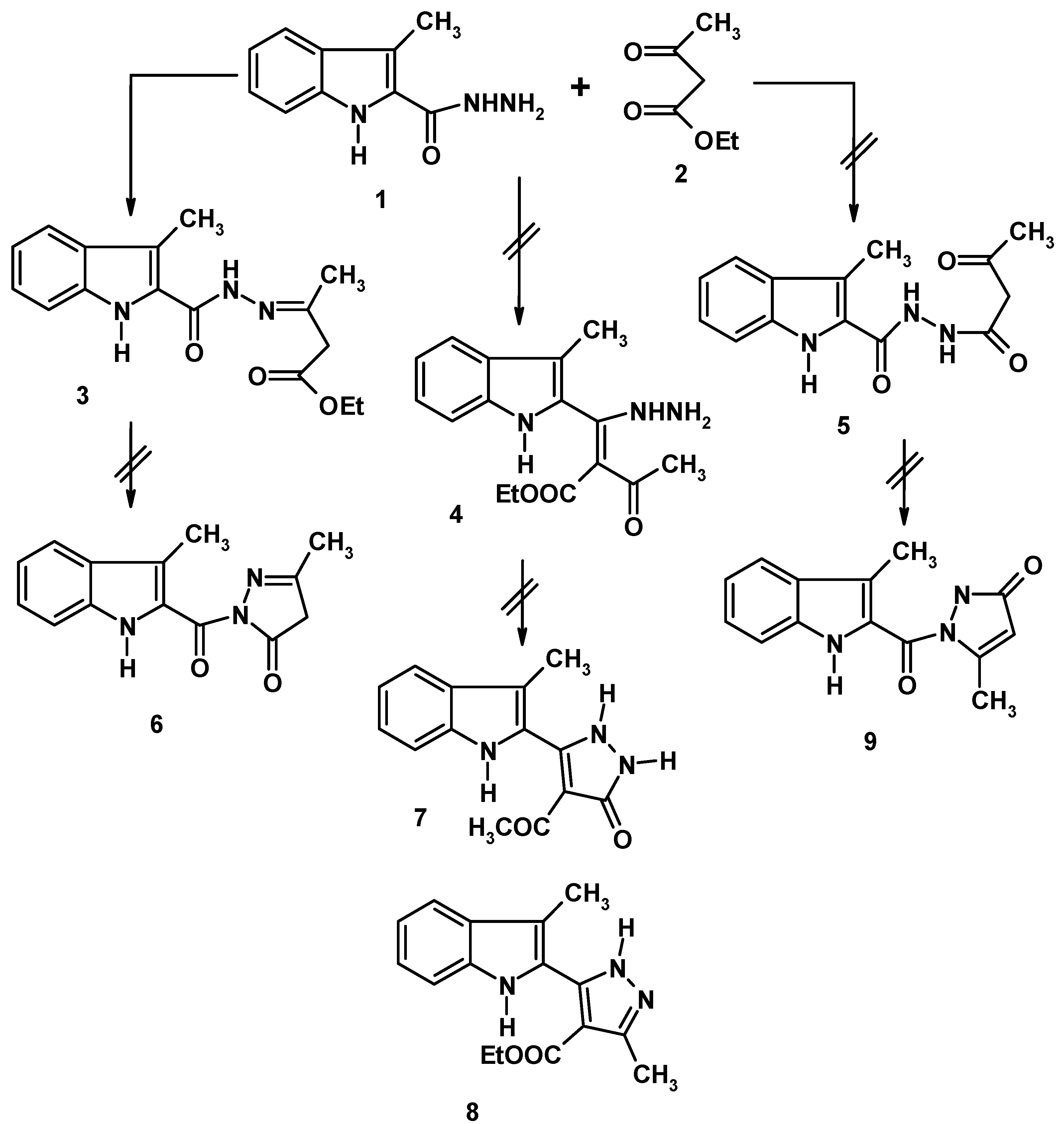
:Results and Discussion
Crystallographic Analysis
Crystal Data for Compound 3
Synthesis of Ethyl 3-{2-[(3-Methyl-1H-indol-2-yl)carbonyl]hydrazinylidene}butanoate (3)
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3References and Notes
- Ismail, M.F.; Shmeiss, N.A.M.M.; El-Diwani, H.I.; Arbid, M.S. Synthesis and pharmacological activity of some 2,3-diphenylindole derivative. Indian J. Chem. Sect. B 1997, 36B, 288–292. [Google Scholar]
- von Angerer, E.; Knebel, N.; Kager, M.; Ganss, B.J. 1-(Aminoalkyl)-2-phenylindoles as novel pure Estrogen Antagonists. J. Med. Chem. 1900, 33, 2635–2640. [Google Scholar] [CrossRef]
- Biberger, C.; Von Angerer, E.J. 1-benzyl-2-phenylindole- and 1,2-diphenylindole-based antiestrogens. Estimation of agonist and anatagonist activities in transfection assays. J. Steroid Biochem. Mol. Bio. 1998, 64, 277–281. [Google Scholar] [CrossRef]
- Stevenson, G.I.; Smith, A.L.; Lewis, S.; Michie, S.G.; Neduvelil, J.G.; Patel, S.; Marwood, R.; Castro, J.L. 2-Aryl tryptamines: Selective high-affinity antagonists for the h5-HT2A receptor. Bioorg. Med. Chem. Lett. 2000, 10, 2697–2699. [Google Scholar] [CrossRef]
- Smith, A.L.; Stevenson, G.I.; Swain, C.J.; Castro, J.L. Tracelless solid phase synthesis of 2,3- disubstituted indoles. Tetrahedron Lett. 1998, 39, 8317–8320. [Google Scholar] [CrossRef]
- Casapullo, A.; Bifulco, G.; Bruno, I.; Riccio, R. New bisindole alkaloids of the Topsentin and Hamacanthin classes from the mediterranean marine sponge Rhaphisia lacazei. J. Nat. Prod. 2000, 63, 447–451. [Google Scholar] [CrossRef] [PubMed]
- Kaniwa, K.; Arai, M.A.; Li, X.; Ishibashi, M. Synthesis, determination of stereochemistry, and evaluation of new bisindole alkaloids from the myxomycete Arcyria ferruginea: An approach for Wnt signal inhibitor. Bioorg. Med. Chem. Lett. 2007, 17, 4254–4257. [Google Scholar] [CrossRef] [PubMed]
- Gribble, G.W.J. Recent developments in indole ring synthesis-methodology and applications. J. Chem. Soc. Perkin Trans. 1 2000, 1045–1075. [Google Scholar] [CrossRef]
- Acheson, R.M.; Prince, R.J.; Proctor, G. Two novel indole rearrangements. J. Chem. Soc. Perkin Trans. 1 1979, 595–598. [Google Scholar] [CrossRef]
- Crystal data for compound 3 ref. CCDC 846358 can be obtained on request from the director, Cambridge Crystallographic Data Center, 12 Union Road, Cambridge CB2 1EW, UK.

| Bond length, Å | Bond length, Å | Bond length, Å |
| C7—C15, 1.472 | N6—C14, 1.286 | C12—C17, 1.517 |
| N5—C7, 1.359 | C14—C21, 1.487 | O1—C17, 1.337 |
| N5—N6. 1.380 | C12—C14, 1.493 | O1—C20, 1.450 |
| Angle (ω) | Angle (ω) | Angle (ω) |
| C17—O1—C20, 115.7 | O2—C7—N5, 117.4 | N23—C10—C8, 108.4 |
| C10—N23—C15, 108.5 | O2—C7—C15, 120.3 | N23—C10—C13, 129.4 |
| N6—N5—C7, 121.0 | N5—C7—C15, 122.3 | C8—C10—C13, 122.2 |
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Farghaly, T.A.; Gomha, S.M. Ethyl 3-{2-[(3-Methyl-1H-indol-2-yl)carbonyl]hydrazinylidene}butanoate. Molbank 2012, 2012, M749. https://doi.org/10.3390/M749
Farghaly TA, Gomha SM. Ethyl 3-{2-[(3-Methyl-1H-indol-2-yl)carbonyl]hydrazinylidene}butanoate. Molbank. 2012; 2012(1):M749. https://doi.org/10.3390/M749
Chicago/Turabian StyleFarghaly, Thoraya A., and Sobhi M. Gomha. 2012. "Ethyl 3-{2-[(3-Methyl-1H-indol-2-yl)carbonyl]hydrazinylidene}butanoate" Molbank 2012, no. 1: M749. https://doi.org/10.3390/M749
APA StyleFarghaly, T. A., & Gomha, S. M. (2012). Ethyl 3-{2-[(3-Methyl-1H-indol-2-yl)carbonyl]hydrazinylidene}butanoate. Molbank, 2012(1), M749. https://doi.org/10.3390/M749