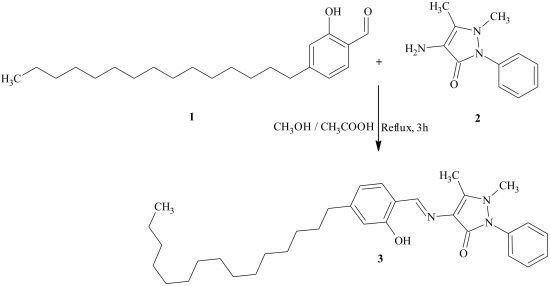
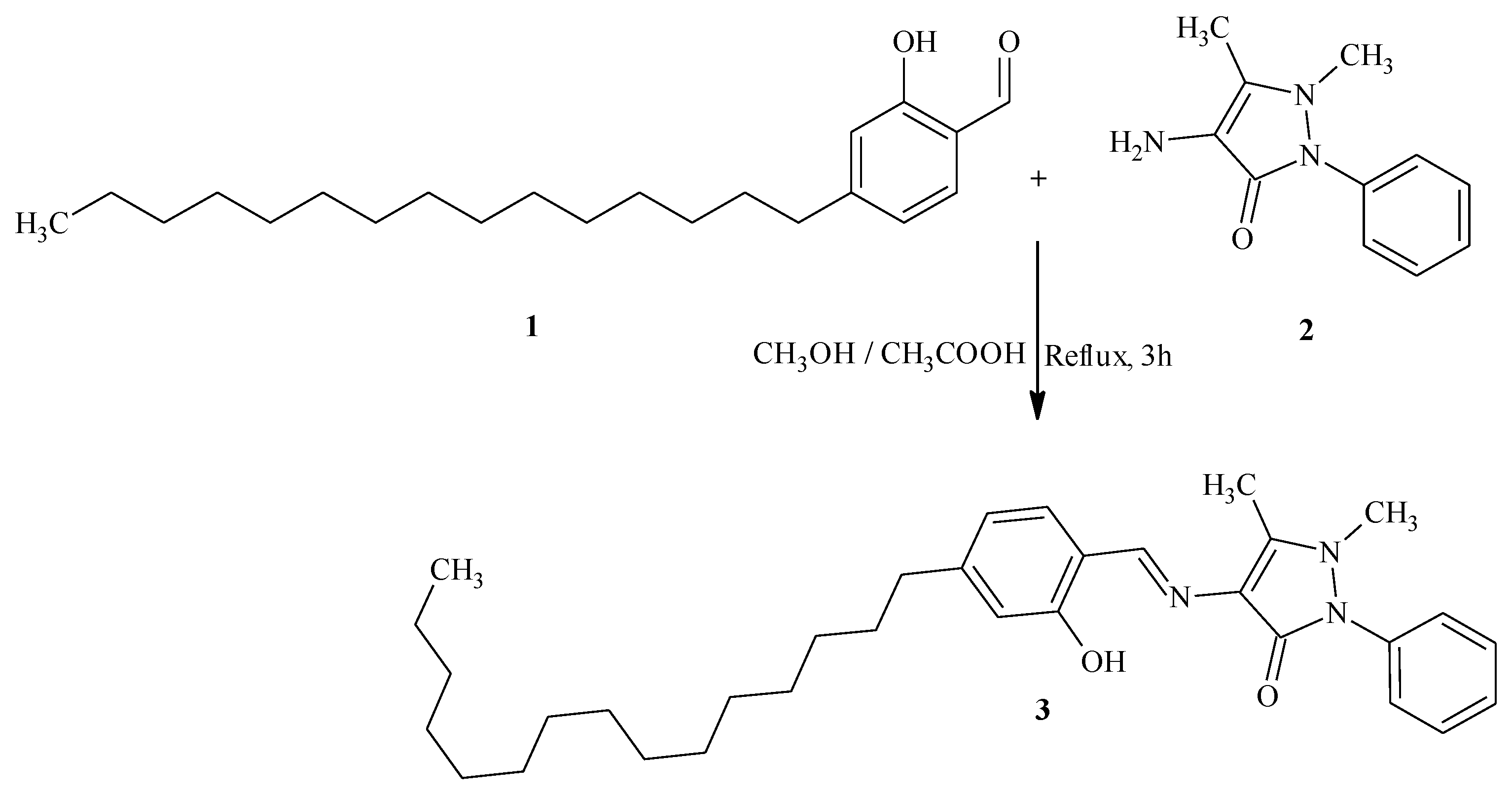
4-[(2-Hydroxy-4-pentadecylbenzylidene)amino]-1,5-dimethyl-2-phenyl-1,2-dihydro-3H-pyrazol-3-one
Abstract
:Introduction
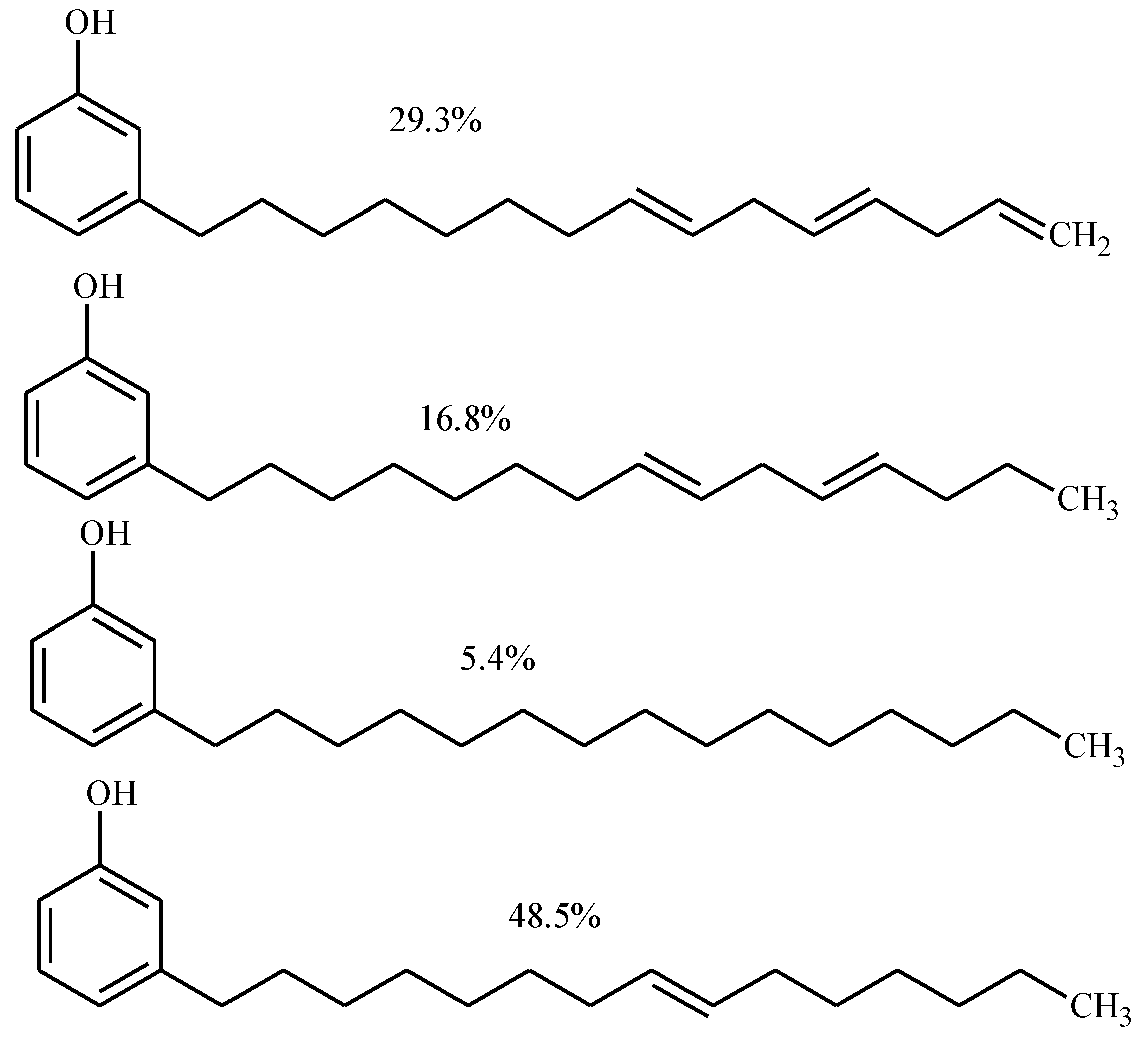
Result and Discussion
Experimental
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3Acknowledgments
References
- Hadi, V.; Koh, Y.-H.; Sanchez, T.W.; Barrios, D.; Neamati, N.; Jung, K.W. Development of the next generation of HIV-1 integrase inhibitors: Pyrazolone as a novel inhibitor scaffold. Bioorg. Med. Chem. 2010, 20, 6854–6857. [Google Scholar] [CrossRef] [PubMed]
- Brana, M.F.; Gradillas, A.; Ovalles, A.G.; Lopez, B.; Acero, N.; Llinares, F.; Mingarro, D.M. Synthesis and biological activity of N,N-dialkylaminoalkyl substituted bisindolyl and diphenyl pyrazolone derivatives. Bioorg. Med. Chem. 2006, 14, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Ismail, M.M.F.; Ammar, Y.A.; El-Zahaby, H.S.A.; Eisa, S.I.; Barakat, S.E. Synthesis of novel 1-pyrazolylpyridin-2-ones as potential anti-inflammatory and analgesic agents. Arch. Pharm. Life Sci. 2007, 340, 476–482. [Google Scholar] [CrossRef] [PubMed]
- El-Hawash, A.M.; El-Sayed, A.M.B.; El-Ashmawey, I.M. Nonsteroidal anti-inflammatory agents-part-2 anti-inflammatory, analgesic and antipyretic activity of some substituted 3-pyrazolin- 5-ones and 1,2,4,5,6,7-3H-hexahydroindazol-3-ones. Eur. J. Med. Chem. 2006, 41, 155–165. [Google Scholar] [CrossRef] [PubMed]
- Manojkumar, P.; Ravi, T.K.; Gopalakrishnan, S. Antioxidant and antibacterial studies of arylazopyrazoles and arylhydrazonopyrazolones containing coumarin moiety. Eur. J. Med. Chem. 2009, 44, 4690–4694. [Google Scholar] [CrossRef] [PubMed]
- Filho, V.C.; Correa, R.; Vaz, Z.; Calixto, J.B.; Nunes, R.J.; Pinheiro, T.R.; Andricopulo, A.D.; Yunes, R.A. Further studies on analgesic activity of cyclic imides. Farmaco 1998, 53, 55–57. [Google Scholar] [CrossRef]
- Gadada, Naganagowda; Amorn, Petsom. 4-Amino-N-(2-hydroxy-4-pentadecylbenzylidene) benzenesulfonamide. Molbank 2011, 2011, M739. [Google Scholar]
- Payne, P.; Tyman, J.H.P.; Mehet, S.K.; Ninagawa, A. The synthesis of 2-hydroxymethyl derivatives of phenols. J. Chem. Res. 2006, 37, 402–405. [Google Scholar] [CrossRef]
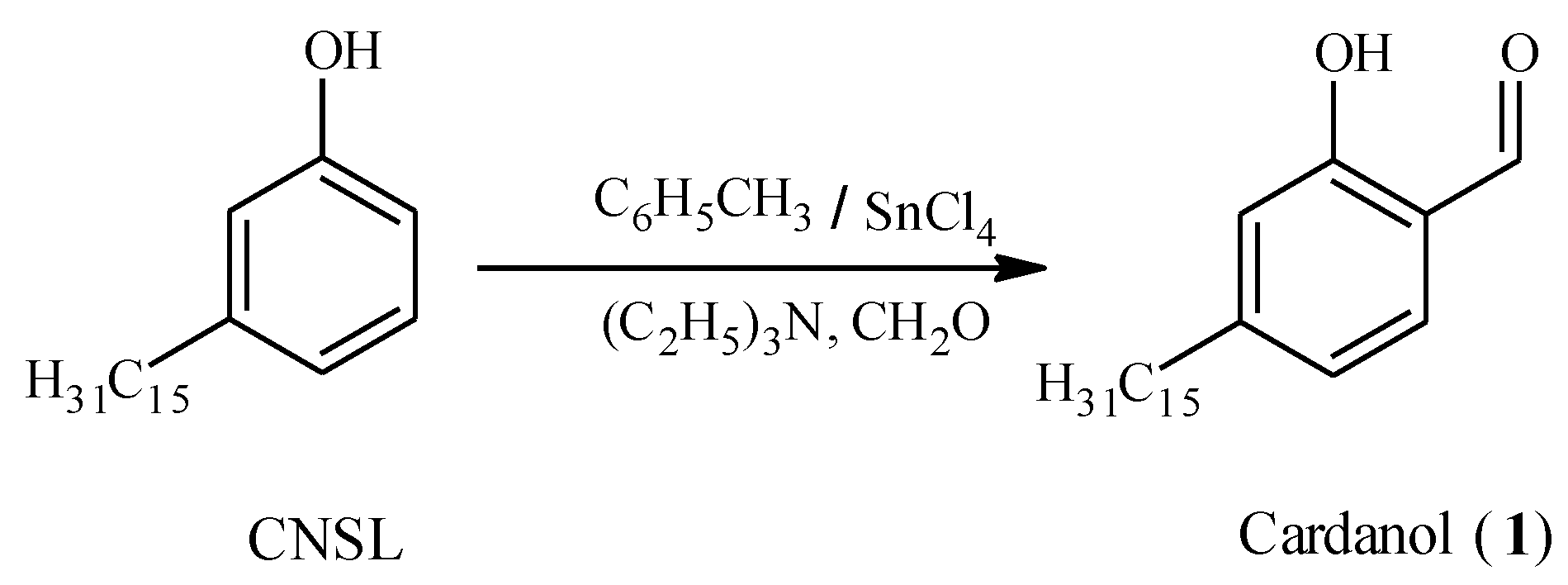
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Naganagowda, G.; Petsom, A. 4-[(2-Hydroxy-4-pentadecylbenzylidene)amino]-1,5-dimethyl-2-phenyl-1,2-dihydro-3H-pyrazol-3-one. Molbank 2012, 2012, M750. https://doi.org/10.3390/M750
Naganagowda G, Petsom A. 4-[(2-Hydroxy-4-pentadecylbenzylidene)amino]-1,5-dimethyl-2-phenyl-1,2-dihydro-3H-pyrazol-3-one. Molbank. 2012; 2012(1):M750. https://doi.org/10.3390/M750
Chicago/Turabian StyleNaganagowda, Gadada, and Amorn Petsom. 2012. "4-[(2-Hydroxy-4-pentadecylbenzylidene)amino]-1,5-dimethyl-2-phenyl-1,2-dihydro-3H-pyrazol-3-one" Molbank 2012, no. 1: M750. https://doi.org/10.3390/M750
APA StyleNaganagowda, G., & Petsom, A. (2012). 4-[(2-Hydroxy-4-pentadecylbenzylidene)amino]-1,5-dimethyl-2-phenyl-1,2-dihydro-3H-pyrazol-3-one. Molbank, 2012(1), M750. https://doi.org/10.3390/M750