Abstract
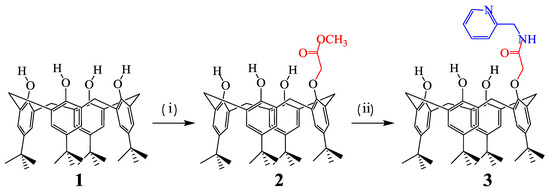
5,11,17,23-tetra-tert-butyl-25-(2’-pyridyl methyl amidocarbonylmethyl)-calix[4]arene (3) has been synthesized in the cône conformation through reaction of the corresponding mono-ester with 2-aminomethylpyridine (picolylamine) and characterised using 1H NMR and MALDI-TOF mass spectral data as well as elemental analyses.
The present manuscript concerns a class of macrocycles widely used as complexing agents and as substrates for the construction of more elaborate receptors with broader applications. These macrocycles are the calix[n]arenes [1,2], macrocycles composed of n phenolic units (n = 4–20) connected by methylene bridges at positions ortho to the hydroxyl function. Their name derives from the perceived resemblance of the smallest calixarene, calix[4]arene, in its so-called cone configuration where all the hydroxyl groups are directed to the same side of the non-planar macrocycle, to the antique Greek vase known as a “calix crater” [3]. Cone calix[4]arene, in particular, may be considered as a ditopic receptor with a hydrophilic site defined by the hydroxyl groups and a hydrophobic cavity defined by the phenyl groups.
In earlier work [4,5], we have described the syntheses of several calixarene derivatives incorporating amido functional groups, including a tetra-amide derivative of calix[4]arene obtained from 2-aminomethylpyridine (picolylamine) which exhibited useful metal-ion binding properties [6]. The present work is part of our extended studies of this system aimed at evaluating the effect of varying the number of amido binding sites attached to the calix[4]arene scaffold.
Experimental
All reagents and solvents for synthesis were commercial and used without further purification. All the reactions were performed under a N2 atmosphere. TLC was carried out on Silica gel 60 F254 (Merck 1.05554.0001). SiO2 (Geduran 1.11567) was used for column chromatography. 1H NMR spectra were recorded using a 300 MHz Bruker SY 200 instrument. Shifts (δ) are referenced relative to internal tetramethylsilane (TMS). Coupling constants J are given in Hertz. MALDI TOF mass spectra were measured on a Biflex Bruker instrument. Melting points were measured using an electrothermal Buchi 535 melting point (Mp) apparatus and are reported uncorrected. Elemental analyses were performed by the Microanalytical Service of the University of Strasbourg. 5,11,17,23-tetra-tert-butyl-25-(methoxycarbonylmethyl)-calix[4]arene 2 was prepared according to the literature [7].
Preparation of 3: 5,11,17,23-tetra-tert-butyl-25-(methoxycarbonylmethyl)-calix[4]arene 2 (0.600 g; 0.90 mmol) was reacted with an excess of 2-(aminomethyl)pyridine (0.94 g; 1.79 mmol) in a refluxing 1:1 mixture of methanol–toluene (6 mL) for 5 days. The solvents were evaporated and the residue was chromatographed on a silica column using CH2Cl2/CH3OH 96/04 as eluent. Pure 3 was obtained as a white solid (143 mg, 20%).
Melting point: 240–242 °C.
MALDI TOF mass spectrum: m/z = 797.80 ([M+H]+).
1H-NMR (300 MHz, CDCl3) (δ/ppm) : 9.72 (t, J = 5.0 Hz, 1H, NH), 9.12 (s, 2H, OH), 8.86 (s, 1H, OH) 8.70 (d, J = 7.5 Hz, 1H, PyH6), 7.72 (t, J = 7.5 Hz, 1H, PyH5), 7.45 (d, J = 7.5 Hz, 1H, PyH3), 7.20 (t, J = 7.5 Hz, 1H, PyH4), 7.10 (s, 2H, ArHmetacalix), 7.07 (s, 4H, ArHmetacalix), 7.00 (d, J = 1.5 Hz, 2H, ArHmetacalix), 4.88 (d, J = 5.5 Hz, 2H, CH2Py), 4.55 (s, 4H, -CH2OAr), 4.25 (AB system, J = 14.0 Hz, 2H, ArCH2Ar), 4.15 (AB system, J = 14.0 Hz, 2H, ArCH2Ar), 3.53 (AB system, J = 14.0 Hz, 2H, ArCH2Ar), 3.45 (AB system, J = 14.0 Hz, 2H, ArCH2Ar), 1.23 (s, 9H, -C(CH3)3), 1.21 (s, 18H, C(CH3)3), 1.18 (s, 9H, -C(CH3)3).
Elemental analysis: Calculated for C52H64O5N2, C 78.35, H, 8.09; Found: C, 78.1, H, 8.08%.
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3Acknowledgments
This work was supported by Chemistry Department, Faculty of Science and Arts at Al-Rass, Qassim University, Kingdom of Saudi Arabia and Laboratoire d’Application de la Chimie aux Ressources et Substances Naturelles et à l’Environnement (LACReSNE), Faculté des Sciences de Bizerte, Tunisia.
References
- Gutsche, C.D.; Stoddart, J.F. (Eds.) Calixarenes—Monographs in Supramolecular Chemistry; The Royal Society of Chemistry: Cambridge, UK, 1989.
- Vicens, J.; Böhmer, V. (Eds.) Calixarènes: A Versatile Class of Macrocyclic Componds; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1991.
- Gutsche, C.D.; Muthukrishnan, R. Calixarenes. 1. Analysis of the product mixtures produced by the base-catalyzed condensation of formaldehyde with para-substituted phenols. J. Org. Chem. 1978, 43, 4905–4911. [Google Scholar] [CrossRef]
- Hamdi, A.; Abidi, R.; Ayadi, M.T.; Thuéry, P.; Nierlich, M.; Asfari, Z.; Vicens, J. Synthesis and cation complexation studies of a new tetra(2-pyridylmethyl)amide calix[4]arene. Tetrahedron Lett. 2001, 42, 3595–3598. [Google Scholar] [CrossRef]
- Hamdi, A.; Abidi, R.; Asfari, Z.; Vicens, J. Synthesis and Complexing Properties of Five Tetraamido-type p-tert-Butyl Calix[4]arenes Presenting Two Proximal Binding Subunits. J. Incl. Phenom. Macro. Chem. 2003, 45, 99–107. [Google Scholar] [CrossRef]
- Roymon, J.; Jugun, P.C.; Chebrolu, P.R. Benzothiazole appended lower rim 1,3-di-amido-derivative of calix[4]arene: Synthesis, structure, receptor properties towards Cu2+, iodide recognition and computational modeling. Inorg. Chim. Acta 2010, 363, 2833–2839. [Google Scholar]
- Groenen, L.C.; Ruël, B.H.M.; Casnati, A.; Verboom, W.; Pochini, A.; Ungaro, R.; Reinhoudt, D.N. Synthesis of monoalkylated calix[4]arenes via direct alkylation. Tetrahedron 1991, 47, 8379–8384. [Google Scholar] [CrossRef]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).