Abstract
Novel (E)-ethyl 3-(dimethylamino)-2-(7,9-diphenyl-7H-pyrazolo[4,3-e][1,2,4]triazolo[1,5-c]pyrimidin-2-yl)acrylate (3A), was prepared via condensation of ethyl (1,3-diphenyl-1H-pyrazolo[4,3-e][1,2,4]triazolo[1,5-c]pyrimidin-5-yl)acetate (1) and dimethylformamide-dimethylacetal under reflux. The structure of the synthesized compound was assigned on the basis of elemental analysis, IR, 1H NMR and mass spectral data.
Enaminones are poly-dentate reagents that have been utilized extensively in this decade as building blocks in organic synthesis [1,2,3,4,5]. Many reports indicated that the presence of a basic side chain like the N,N-dialkylaminoalkyl group enhances the drugs’ DNA affinity [6] and their anticancer activity, e.g., against the human breast cancer cell line, MCF-7 [7]. In addition, pyrazolo[4,3-e][1,2,4]triazolo[1,5-c]pyrimidines have been used as potent and selective adenosine A2A receptor antagonists [8,9,10,11,12,13,14,15]. This finding prompted us to condense ethyl (1,3-diphenyl-1H-pyrazolo[4,3-e][1,2,4]triazolo[1,5-c]pyrimidin-5-yl)acetate (1) [16] with dimethylformamide-dimethylacetal (DMF-DMA) to obtain the title compound 3A (Scheme 1). Elemental analyses and spectral data were in complete accordance with the assigned structure 3. For example, the 1H NMR spectrum of compound 3 revealed two singlet signals at δ 3.30 and 7.77 ppm characteristic for N,N-dimethylamino and the exocyclic C=CH protons, respectively [1]. The value of the exocyclic C=CH proton signal at δ 7.77 ppm correlated with the E-isomer (3A), while the Z-isomer 3B of an analogous structure was reported to appear at δ 6.9 ppm [17]. Thus, we have successfully synthesized a new enaminone in good yield which can be used as a building blocks for heterocyclic ring systems in organic synthesis.
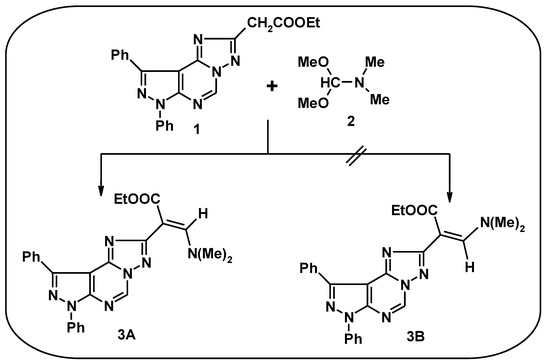
Scheme 1.
Synthesis of the title compound (3A).
Synthesis of (E)-ethyl 3-(dimethylamino)-2-(7,9-diphenyl-7H-pyrazolo[4,3-e][1,2,4]triazolo[1,5-c]pyrimidin-2-yl)acrylate (3)
A mixture of 1 (4.3 g, 10 mmol) and dimethylformamide-dimethylacetal (10 mL) was refluxed for 20 min. After cooling, the precipitate was collected by filtration, washed with methanol and crystallized from dioxane.
Yield: 92%; pale yellow crystals; m.p. 240 °C.
GC-MS m/z (%): 454 (M++ 1, 4), 453 (M+, 13), 380 (11), 336 (12), 167 (23),77 (100), 51 (48).
IR (KBr) vmax cm−1: 1624 (C=O).
1H NMR (Bruker, 300 MHz, CDCl3): δ (ppm) = 1.13 (t, J = 7.0 Hz, 3H, CH3), 3.30 (s, 6H, 2 CH3), 4.07 (q, J = 7.0 Hz, 2H, CH2), 7.47-8.7 (m, 10H, Ar-H), 7.77 (s, 1H, =CH), 9.68 (s, 1H, pyrimidine-H).
Anal. Calcd. for C25H23N7O2 (453.19): C, 66.21; H, 5.11; N, 21.62. Found: C, 66.11; H, 5.01; N, 21.39%.
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3References
- Farghaly, T.A.; Abdel Hafez, N.A.; Ragab, E.A.; Awad, H.M.; Abdalla, M.M. Synthesis, anti-HCV, antioxidant, and peroxynitrite inhibitory activity of fused benzosuberone derivatives. Eur. J. Med. Chem. 2010, 45, 492–500. [Google Scholar] [CrossRef] [PubMed]
- Shawali, A.S.; Farghaly, T.A.; Al-Dahshoury, A.R. Synthesis, reactions and antitumor activity of new β-aminovinyl 3- pyrazolyl ketones. ARKIVOC 2009, xiv, 88–89. [Google Scholar]
- Farghaly, T.A.; Abdalla, M.M. Synthesis, tautomerism, and antimicrobial, anti-HCV, anti-SSPE, antioxidant, and antitumor activities of arylazobenzosuberones. Bioorg. Med. Chem. 2009, 17, 8012–8019. [Google Scholar] [CrossRef] [PubMed]
- Farghaly, T.A.; Riyadh, S.M. Microwave assisted synthesis of annelated benzosuberone as new penta-heterocyclic ring systems. ARKIVOC 2009, x, 54–63. [Google Scholar]
- Riyadh, S.M.; Farghaly, T.A.; Abdallah, M.A.; Abdalla, M.M.; Abd El-Aziz, M.R. New pyrazoles incorporating pyrazolylpyrazole moiety: Synthesis, anti-HCV and antitumor activity. Eur. J. Med. Chem. 2010, 45, 1042–1050. [Google Scholar] [CrossRef] [PubMed]
- Ducrocq, C.; Wendling, F.; Tourbez-Perrin, M.; Rivalle, C.; Tambourin, P.; Pochon, F.; Bisagni, E.; Chermann, J.C. Structure-activity relationship in a series of newly synthesized 1-amino-substituted ellipticine derivatives. J. Med. Chem. 1980, 23, 1212. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.S.M.; Farghaly, T.A. Synthesis and reactions of 3-hydrazino-2,7,8,9-tetrahydro-1H-benzo[6′,7′]cyclohepta[1′,2′:4,5]pyrido[2,3-d]pyrimidin-1-one. ARKIVOC 2009, xiii, 31–41. [Google Scholar]
- Ongini, E.; Monopoli, A.; Cacciari, B.; Baraldi, P.G. Selective adenosine A2A receptor antagonists. Il Farmaco 2001, 56, 87–90. [Google Scholar] [CrossRef]
- Kishore, D.P.; Balakumar, C.; Rao, A.R.; Roy, P.P.; Roy, K. QSAR of adenosine receptor antagonists: Exploring physicochemical requirements for binding of pyrazolo[4,3-e]-1,2,4-triazolo[1,5-c]pyrimidine derivatives with human adenosine A3 receptor subtype. Bioorg. Med. Chem. Let. 2011, 21, 818–823. [Google Scholar] [CrossRef] [PubMed]
- Baraldi, P.G.; Manfredini, S.; Simoni, D.; Zappaterra, L.; Zocchi, C.; Dionisotti, S.; Ongini, E. Synthesis of new pyrazolo[4,3-e]1,2,4-triazolo[1,5-c]pyrimidine and 1,2,3-triazolo[4,5-e]1,2,4-triazolo[1,5-c]pyrimidine displaying potent and selective activity as A2a adenosine receptor antagonists. Bioorg. Med. Chem. Lett. 1994, 4, 2539–2544. [Google Scholar] [CrossRef]
- Kumar, T.S.; Mishra, S.; Deflorian, F.; Yoo, L.S.; Phan, K.; Kecskés, M.; Szabo, A.; Shinkre, B.; Gao, Z.-G.; Trenkle, W.; Jacobson, K.A. Molecular probes for the A2A adenosine receptor based on a pyrazolo[4,3-e][1,2,4]triazolo[1,5-c]pyrimidin-5-amine scaffold. Bioorg. Med. Chem. Lett. 2011, 21, 2740–2745. [Google Scholar] [CrossRef] [PubMed]
- Harris, J.M.; Neustadt, B.R.; Zhang, H.; Lachowicz, J.; Cohen-Williams, M.; Varty, G.; Hao, J.; Stamford, A.W. Potent and selective adenosine A2A receptor antagonists: [1,2,4]Triazolo[4,3-c]pyrimidin-3-ones. Bioorg. Med. Chem. Lett. 2011, 21, 2497–2501. [Google Scholar] [CrossRef] [PubMed]
- Baraldi, P.G.; Bovero, A.; Fruttarolo, F.; Romagnoli, R.; Tabrizi, M.A.; Preti, D.; Varani, K.; Borea, P.A.; Moorman, A.R. New strategies for the synthesis of A3 adenosine receptor antagonists. Bioorg. Med. Chem. 2003, 11, 4161–4169. [Google Scholar] [CrossRef]
- Michielan, L.; Bolcato, C.; Federico, S.; Cacciari, B.; Bacilieri, M.; Klotz, K.-N.; Kachler, S.; Pastorin, G.; Cardin, R.; Sperduti, A.; Spalluto, G.; Moro, S. Combining selectivity and affinity predictions using an integrated Support Vector Machine (SVM) approach: An alternative tool to discriminate between the human adenosine A2A and A3 receptor pyrazolo-triazolo-pyrimidine antagonists binding sites. Bioorg. Med. Chem. 2009, 17, 5259–5274. [Google Scholar] [CrossRef] [PubMed]
- Paeshuyse, J.; Letellier, C.; Froeyen, M.; Dutartre, H.; Vrancken, R.; Canard, B.; De Clercq, E.; Gueiffier, A.; Teulade, J.-C.; Herdewijn, P.; Puerstinger, G.; Koenen, F.; Kerkhofs, P.; Baraldi, P.G.; Neyts, J. A pyrazolotriazolopyrimidinamine inhibitor of bovine viral diarrhea virus replication that targets the viral RNA-dependent RNA polymerase. Antivir. Res. 2009, 82, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Farghaly, T.A.; Gomha, S.M. Synthesis of ethyl (1,3-diphenyl-1H-pyrazolo[4,3-e][1,2,4] triazolo[1,5-c]pyrimidin-5-yl)acetate. Molbank 2011, 2011, M743. [Google Scholar] [CrossRef]
- Bennett, P.; Donnelly, J.A.; Meaney, D.C.; Boyle, P.O. Stereochemistry of cyclopropyl ketones from the reaction of dimethylsulphoxonium methylide with 3-benzylidenechroman-4-ones. J. Chem. Soc. Perkin Trans. 1 1972, 1554. [Google Scholar] [CrossRef]
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).