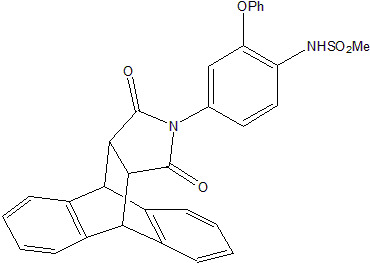
N-(4-Methylsulfonamido-3-phenoxyphenyl)-9,10-dihydro-9,10-ethanoanthracene-11,12-dicarboximide
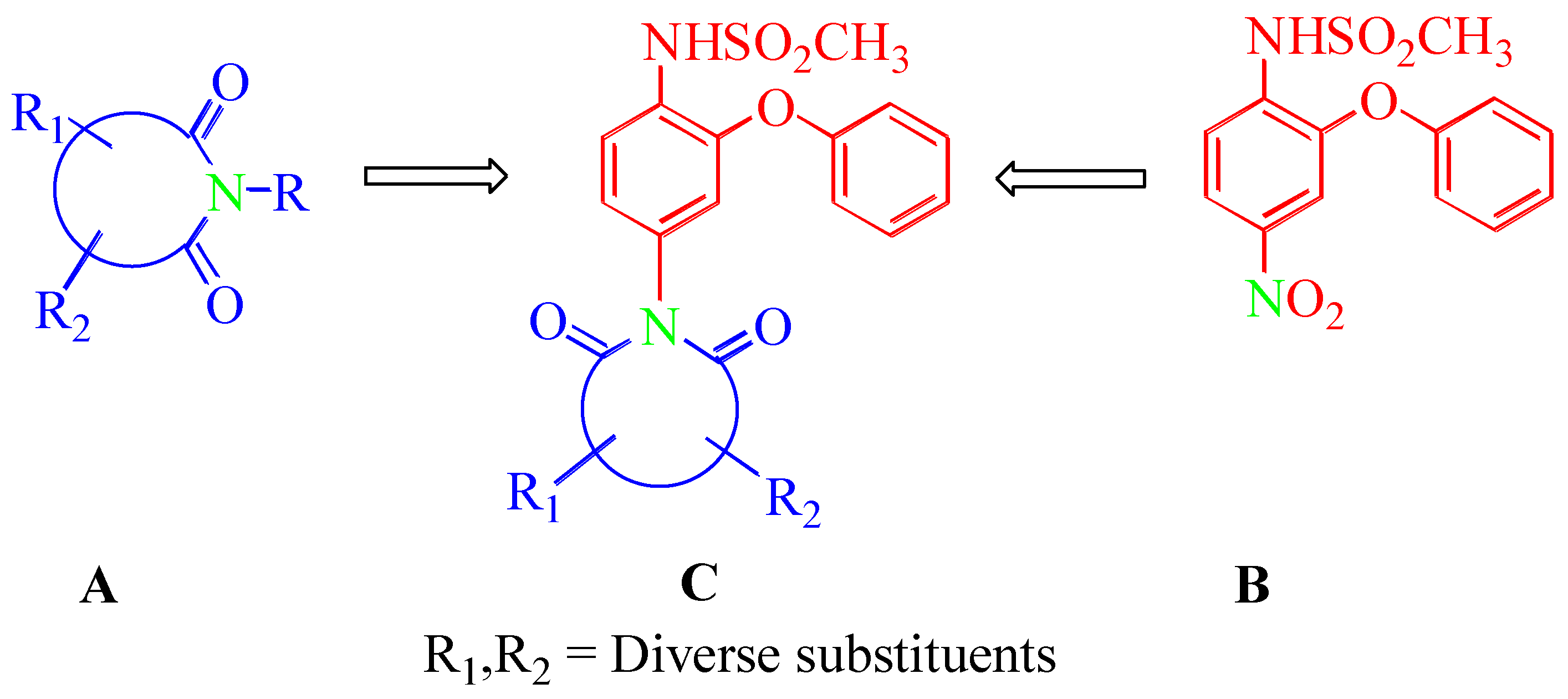
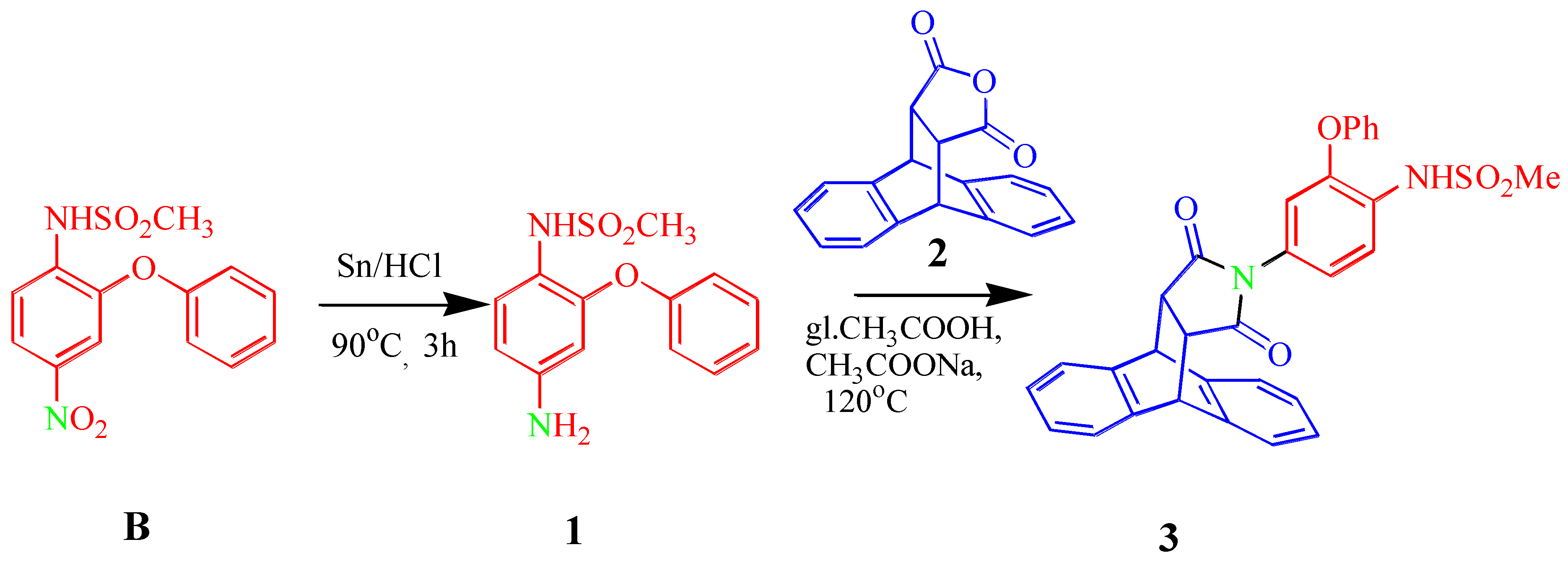
Abstract
:Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3Supplementary File 4Acknowledgments
References and Notes
- Salvati, M.E.; Balog, A.; Shan, W.; Wei, D.D.; Pickering, D.; Attar, R.M.; Geng, J.; Rizzo, C.A.; Gottardis, M.M.; Weinmann, R.; Krystek, S.R.; Sack, J.; An, Y.; Kish, K. Structure based approach to the design of bicyclic-1H-isoindole-1,3(2H)-dione based androgen receptor antagonists. Bioorg. Med. Chem. Lett. 2005, 15, 271–276. [Google Scholar] [CrossRef] [PubMed]
- Sondhi, S.M.; Rani, R.; Roy, P.; Agarwal, S.K.; Saxena, A.K. Microwave-Assisted Synthesis of N-Substituted Cyclic Imides and Their Evaluation for Anticancer and Antiinflammatory Activities. Bioorg. Med. Chem. Lett. 2009, 19, 1534–1538. [Google Scholar] [CrossRef] [PubMed]
- Ishizumi, K.; Kojima, A.; Antoku, F. Synthesis and anxiolytic activity of N-substituted cyclic imides (1R*,2S*,3R*,4S*)-N-[4-[4-(2-pyrimidinyl)-1-piperazinyl]butyl]-2,3-bicyclo[2.2.1] heptanedicarboximide (tandospirone) and related compounds. Chem. Pharm. Bull. 1991, 39, 2288–2300. [Google Scholar] [CrossRef] [PubMed]
- Shibata, Y.; Shiehita, M.; Sasaki, K.; Nishimura, K.; Hashimoto, Y.; Iwasaki, S. N-Alkylphthalimides: Structural requirement of thalidomidal action on 12-O-tetradecanoylphorbol-13-acetate-induced tumor necrosis factor alpha production by human leukemia HL-60 cells. Chem. Pharm. Bull. 1995, 43, 177–179. [Google Scholar] [CrossRef] [PubMed]
- Jindal, D.P.; Bedi, V.; Jit, B.; Karkra, N.; Guleria, S.; Bansal, R.; Palusczak, A.; Hartmann, R.W. Synthesis and study of some new N-substituted imide derivatives as potential anticancer agents. Il Farmaco 2005, 60, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.J.; Wang, S.S.; Lee, C.F.; Chung, M.A.; Chern, Y.T. In vitro antitumor and antimicrobial activities of N-substituents of maleimide by adamantane and diamantane. Chemotherapy 1997, 43, 182–189. [Google Scholar] [CrossRef] [PubMed]
- Machado, A.L.; Lima, L.M.; Arau´jo, J.X., Jr.; Fraga, C.A.M.; Koatz, V.L.G.; Barreiro, E.J.I. Design, synthesis and antiinflammatory activity of novel phthalimide derivatives, structurally related to thalidomide. Bioorg. Med. Chem. Lett. 2005, 15, 1169–1172. [Google Scholar] [CrossRef] [PubMed]
- Kenji, S.; Hideko, N.; Yoshihiro, U.; Yoshikazu, S.; Kazuharu, N.; Motoji, W.; Konstanty, W.; Tadafumi, T.; Tetsuji, A.; Yuji, Y.; Kenji, K.; Hitoshi, H. Napthalimidobenzamide DB-51630: A novel DNA binding agent inducing p300 gene expression and exerting a potent anti-cancer activity. Bioorg. Med. Chem. 2005, 13, 4014–4021. [Google Scholar]
- Mayer, A.; Neuenhofer, S. Luminescent labels - more than just an alternative to radioisotopes. Angew. Chem. Int. Ed. 1994, 33, 1044–1072. [Google Scholar] [CrossRef]
- Miguel, F.B.; Gema, D.; Beatriz, S.; Cynthia, R.; Simmon, R.; Teresa, B. Synthesis and antitumour activity of new dendritic polyamines–(imide–DNA-intercalator) conjugates: potent Lck inhibitors. Eur. J. Med. Chem. 2002, 37, 541–551. [Google Scholar]
- Miyachi, H.; Azuma, A.; Ogasawara, A.; Uchimura, E.; Watanabe, N.; Kobayashi, Y.; Kato, F.; Kato, M.; Hashimoto, H. Novel biological response modifiers: Phthalimides with tumor necrosis factor-alpha production-regulating activity. J. Med. Chem. 1997, 40, 2858–2865. [Google Scholar] [CrossRef] [PubMed]
- Mederski, W.W.K.R.; Baumgarth, M.; Germann, M.; Kux, D.; Weitzel, T. A Convenient Synthesis of 4-Aminoaryl Substituted Cyclic Imides. Tetrahedron Lett. 2003, 44, 2133–2136. [Google Scholar] [CrossRef]
- Cremlyn, R.; Swinbourne, F.; Nunes, R. Diels Alder Reactions using N-(p-Chlorosulfonyl)-Maleimide a dienophile. Phosphorous Sulfur Relat. Elem. 1987, 33, 65. [Google Scholar] [CrossRef]
- Pericherla, S.; Mareddy, J.; Rani, D.P.G.; Gollapudi, P.V.; Pal, S. Chemical Modifications of Nimesulide. J. Braz. Chem. Soc. 2007, 18, 384–390. [Google Scholar] [CrossRef]
- Reddy, L.V.; Nakka, M.; Suman, A.; Ghosh, S.; Helliwell, M.; Mukkanti, K.; Mukherjee, A.K.; Pal, S. Synthesis of Novel Quinoline Analogues of Nimesulide: An Unusual Observation. J. Heterocycl. Chem. 2011, 48, 555–562. [Google Scholar]
- Durgadas, S.; Chatare, V.K.; Mukkanti, K.; Pal, S. Palladium-mediated synthesis of novel nimesulide derivatives. Appl. Organomet. Chem. 2010, 24, 680–684. [Google Scholar] [CrossRef]
- Kankanala, K.; Reddy, V.R.; Mukkanti, K.; Pal, S. Lewis Acid Free High Speed Synthesis of Nimesulide-Based Novel N-Substituted Cyclic Imides. J. Braz. Chem. Soc. 2010, 21, 1060–1064. [Google Scholar] [CrossRef]
- Reddy, L.V.; Kethavath, M.; Nakka, M.; Beevi, S.S.; Mangamoori, L.N.; Mukkanti, K.; Pal, S. Design and Synthesis of Novel Cytotoxic Agents Based on Combined Framework of Quinoline and Nimesulide. J. Heterocycl. Chem. 2011. [CrossRef]
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Kankanala, K.; Prakash, V.; Mukkanti, K.; Reddy, V.R.; Pal, S. N-(4-Methylsulfonamido-3-phenoxyphenyl)-9,10-dihydro-9,10-ethanoanthracene-11,12-dicarboximide. Molbank 2011, 2011, M740. https://doi.org/10.3390/M740
Kankanala K, Prakash V, Mukkanti K, Reddy VR, Pal S. N-(4-Methylsulfonamido-3-phenoxyphenyl)-9,10-dihydro-9,10-ethanoanthracene-11,12-dicarboximide. Molbank. 2011; 2011(4):M740. https://doi.org/10.3390/M740
Chicago/Turabian StyleKankanala, Kavitha, Varsha Prakash, Khagga Mukkanti, Vangala Ranga Reddy, and Sarbani Pal. 2011. "N-(4-Methylsulfonamido-3-phenoxyphenyl)-9,10-dihydro-9,10-ethanoanthracene-11,12-dicarboximide" Molbank 2011, no. 4: M740. https://doi.org/10.3390/M740
APA StyleKankanala, K., Prakash, V., Mukkanti, K., Reddy, V. R., & Pal, S. (2011). N-(4-Methylsulfonamido-3-phenoxyphenyl)-9,10-dihydro-9,10-ethanoanthracene-11,12-dicarboximide. Molbank, 2011(4), M740. https://doi.org/10.3390/M740