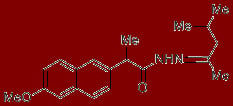
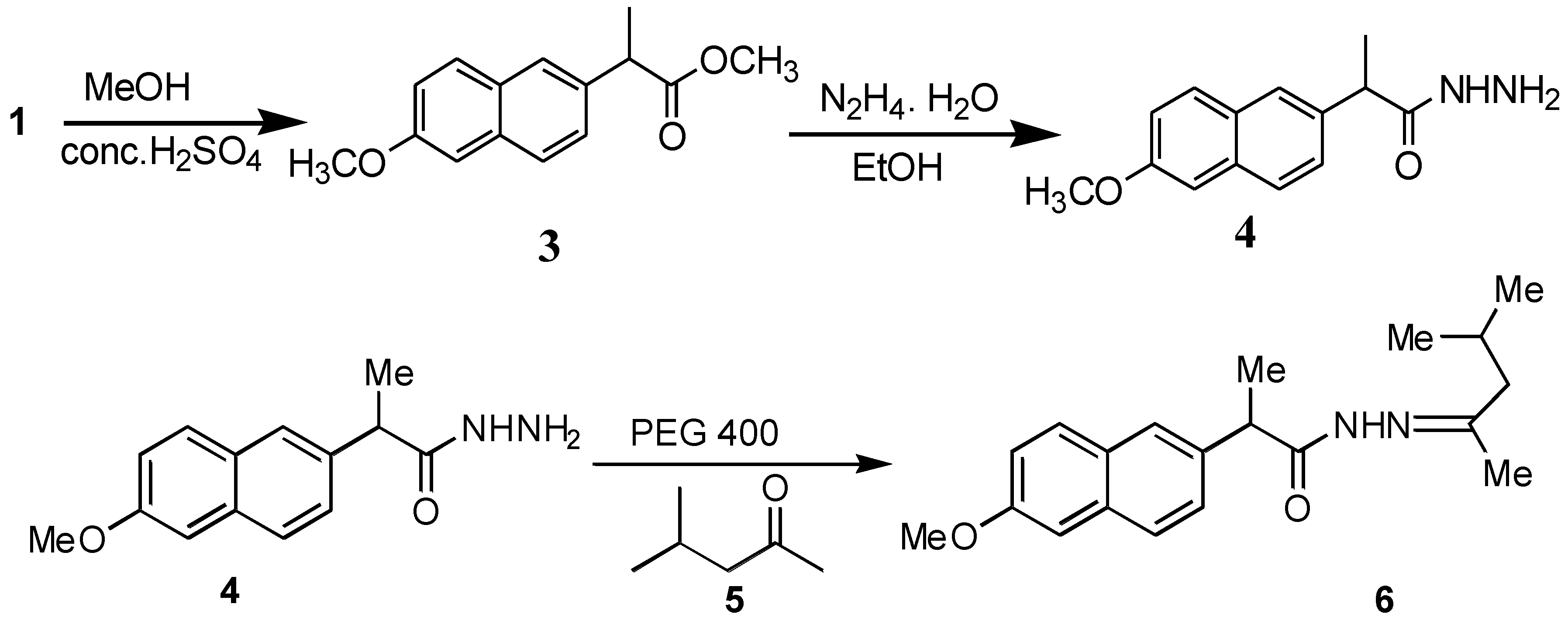
2-(6-Methoxynaphthalen-2-yl)propionic acid (1,3-dimethylbutylidene)hydrazide
Abstract
:
| Code | Eschirichia Coli | Klebsiella pneumonia | Bacillus subtillis | Staphylococcus aureus | Staphylococcus epidermis |
| 6 | +++ | +++ | +++ | ++ | ++ |
| Control | ++++ | ++++ | ++++ | ++++ | ++++ |
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3Acknowledgments
References
- Harrington, P.J.; Lodewijk, E. Large-scale synthetic process for (S)-naproxen by Syntex. Org. Process Res. Dev. 1997, 1, 72–76. [Google Scholar] [CrossRef]
- Rollas, S.; Kucukguzel, S.G. Biological Activities of Hydrazone Derivatives. Molecules 2007, 12, 1910–1939. [Google Scholar] [CrossRef] [PubMed]
- Nayyar, A.; Jain, R. Recent advances in new structural classes of anti-tuberculosis agents. Curr. Med. Chem. 2005, 12, 1873–1886. [Google Scholar] [CrossRef] [PubMed]
- Scior, T.; Garces-Eisele, S.J. Isoniazid is not a lead compound for its pyridyl ring derivatives, isonicotinoyl amides, hydrazides, and hydrazones. Curr. Med. Chem. 2006, 13, 2205–2219. [Google Scholar] [CrossRef] [PubMed]
- Janin, Y. Antituberculosis drugs: Ten years of research. Bioorg. Med. Chem. 2007, 15, 2479–2513. [Google Scholar] [CrossRef] [PubMed]
- Sriram, D.; Yogeeswari, P.; Devakaram, R.V. Synthesis, in vitro and in vivo antimycobacterial activities of diclofenac acid hydrazones and amides. Bioorg. Med. Chem. 2006, 14, 3113–3118. [Google Scholar] [CrossRef] [PubMed]
- Munoz-Muniz, O.; Juaristi, E. Enantioselective protonation of prochiral enolates in the asymmetric synthesis of (S)-naproxen. Tetrahedron Lett. 2003, 44, 2023–2026. [Google Scholar] [CrossRef]
- Mamatha, N.; Murtuja, S.B.; Mathews, V.B.F.; Reddy, L.V.; Bhattacharya, A.; Madeleine, H.; Alok, K.M.; Syed, S.B.; Lakshmi, N.M.; Mukkanti, K.; Pal, S. Naproxen and ibuprofen based acyl hydrazone derivatives: Synthesis, structure analysis and cytotoxicity studies. J. Chem. Pharm. Res. 2010, 2, 393–409. [Google Scholar]

© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Mamatha, N.; Babu, N.S.; Mukkanti, K.; Pal, S. 2-(6-Methoxynaphthalen-2-yl)propionic acid (1,3-dimethylbutylidene)hydrazide. Molbank 2011, 2011, M741. https://doi.org/10.3390/M741
Mamatha N, Babu NS, Mukkanti K, Pal S. 2-(6-Methoxynaphthalen-2-yl)propionic acid (1,3-dimethylbutylidene)hydrazide. Molbank. 2011; 2011(4):M741. https://doi.org/10.3390/M741
Chicago/Turabian StyleMamatha, Nakka, Nallapati Suresh Babu, Khagga Mukkanti, and Sarbani Pal. 2011. "2-(6-Methoxynaphthalen-2-yl)propionic acid (1,3-dimethylbutylidene)hydrazide" Molbank 2011, no. 4: M741. https://doi.org/10.3390/M741
APA StyleMamatha, N., Babu, N. S., Mukkanti, K., & Pal, S. (2011). 2-(6-Methoxynaphthalen-2-yl)propionic acid (1,3-dimethylbutylidene)hydrazide. Molbank, 2011(4), M741. https://doi.org/10.3390/M741